| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 11, November 2024, pages 347-353
A Rare Case of Systemic Epstein-Barr Virus-Positive Diffuse Large B-Cell Lymphoma With Hemophagocytic Lymphohistiocytosis in an Immunocompetent Young Man: Potential Diagnostic Pitfall and Therapeutic Challenge
Shu Yao Liua , Sha Zhaob, Yu Wuc, Guang Cui Hed, e
aSchool of Clinical Medicine, Chengdu Medical College, Chengdu 610500, Sichuan, China
bDepartment of Pathology, West China Hospital, Sichuan University, Chengdu 610041, Sichuan, China
cDepartment of Hematology, West China Hospital, Sichuan University, Chengdu 610041, Sichuan, China
dDepartment of Hematology, The General Hospital of Western Theater Command, PLA, Chengdu 610083, Sichuan, China
eCorresponding Author: Guang Cui He, Department of Hematology, The General Hospital of Western Theater Command, PLA, Chengdu 610083, Sichuan, China
Manuscript submitted August 19, 2024, accepted September 18, 2024, published online October 10, 2024
Short title: Systemic EBV+ DLBCL With HLH
doi: https://doi.org/10.14740/jmc4314
| Abstract | ▴Top |
Epstein-Barr virus-positive diffuse large B-cell lymphoma (EBV+ DLBCL) is an uncommon subtype of aggressive B-cell lymphoma, with both nodal and extranodal involvement being exceedingly rare. We present a unique case of a 32-year-old immunocompetent male with a nasopharynx lesion accompanied by fever and bilateral cervical lymphadenopathy. The initial biopsy from the nasopharynx proposed infectious mononucleosis (IM) as a potential diagnostic pitfall. The further discovery of lymph node and intestinal mucosa biopsies confirmed the diagnosis of systemic EBV+ DLBCL. After receiving four cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) treatment, the patient got complete remission. However, hemophagocytic lymphohistiocytosis (HLH) developed following the fifth cycle of CHOP. The patient accepted allogeneic hematopoietic stem cell transplantation (allo-HCT) subsequently. Unfortunately, the survival time was only 14 months. Appeals for a multi-dimension approach to understanding more fully and improving the outcomes of such cases are underscored.
Keywords: Epstein-Barr virus-positive diffuse large B-cell lymphoma; Systemic; Hemophagocytic lymphohistiocytosis; Immunocompetent; Young adult; Diagnostic pitfall; Therapeutic challenge
| Introduction | ▴Top |
Epstein-Barr virus (EBV)-associated B-cell lymphoproliferative disorders (LPDs) incorporate diseases with an extensively clinicopathological spectrum spanning from self-limiting, indolent, and localized lesions to highly aggressive lymphomas, including infectious mononucleosis (IM) and EBV-positive diffuse large B-cell lymphoma (EBV+ DLBCL) [1]. Some of EBV-positive B-cell LPDs occur in context of inborn or acquired immune deficiency/dysregulation (IDD) or immunosenescence [2].
In 2008, the WHO classification of lymphoid tissue neoplasms introduced EBV+ DLBCL of the elderly as a tentative entity [3]. Later in the 2016 revision of the WHO classification, it was redefined as EBV+ DLBCL, not otherwise specified (NOS) [4]. Then it was revised again in the fifth edition of WHO classification (WHO-HEMA5) as EBV+ DLBCL in 2022 [2]. EBV+ DLBCL is unusual. Compared to the prevalence of 4.7% in Western countries, the rate was markedly higher in Asia and South America at 9.2% [5]. While elderly patients show a higher frequency of extranodal manifestations, younger patients who are less than 45 years old, often acquire predominantly nodal involvements. Both nodal and extranodal involvements were reported in about 5-10% of the patients [2].
Hemophagocytic lymphohistiocytosis (HLH) is a rare but lethal hyperinflammatory syndrome [6], which is particularly triggered by EBV infection. HLH has been traditionally classified into primary and secondary forms. Primary HLH refers to familial autosomal recessive and the secondary one has to do with infections, autoimmune and metabolic disorders, malignancy, transplantations, and so on [7]. Secondary HLH can occur not only in lymphoma onset, but also in time of relapsing or advancing lymphoma [8].
We herein present a rare EBV+ DLBCL in a young man, which systematically involved lymph nodes and extranodal sites (nasopharynx, gastrointestinal (GI) tract). No underlying immune deficiency or dysregulation was identified. The patient later developed HLH which brought tough therapeutic challenge.
| Case Report | ▴Top |
A 32-year-old Chinese man presented with a 1-month history of recurrent cough, stinky runny nose, and nasal discomfort, accompanied by fever. The past medical history was not remarkable. A nasopharynx mass was found by nasopharyngoscopy examination. Histology of the biopsy specimen showed diffuse proliferation of pleomorphic medium-sized or large lymphoid cells in laminae propria mucosae. Immunohistochemical staining showed that CD20, CD79a, BCL6, MUM1, and BCL2 were positive in these lymphoid cells. Ki-67 index was 80%. In situ hybridization (ISH) for EBV-encoded small RNA (EBER) was positive (Fig. 1). Clonal amplification peaks of IGH and IGK genes were observed in gene rearrangements. EBV+ DLBCL was the first choice of diagnosis. However, the differential diagnosis is challenging, particularly regarding the overlap with IM in such an immunocompetent young adult. The distinction should be made on the basis of the clinical context. The patient’s serum lactate dehydrogenase (LDH) was elevated (549 IU/L, reference: 120 - 250 IU/L). Plasma EBV-DNA quantification revealed 1.66 × 104 copies/mL. Except a slight decrease of IgM (331 mg/L, normal range 400 - 2,300 mg/L), the rest of immunoglobulin levels were normal.
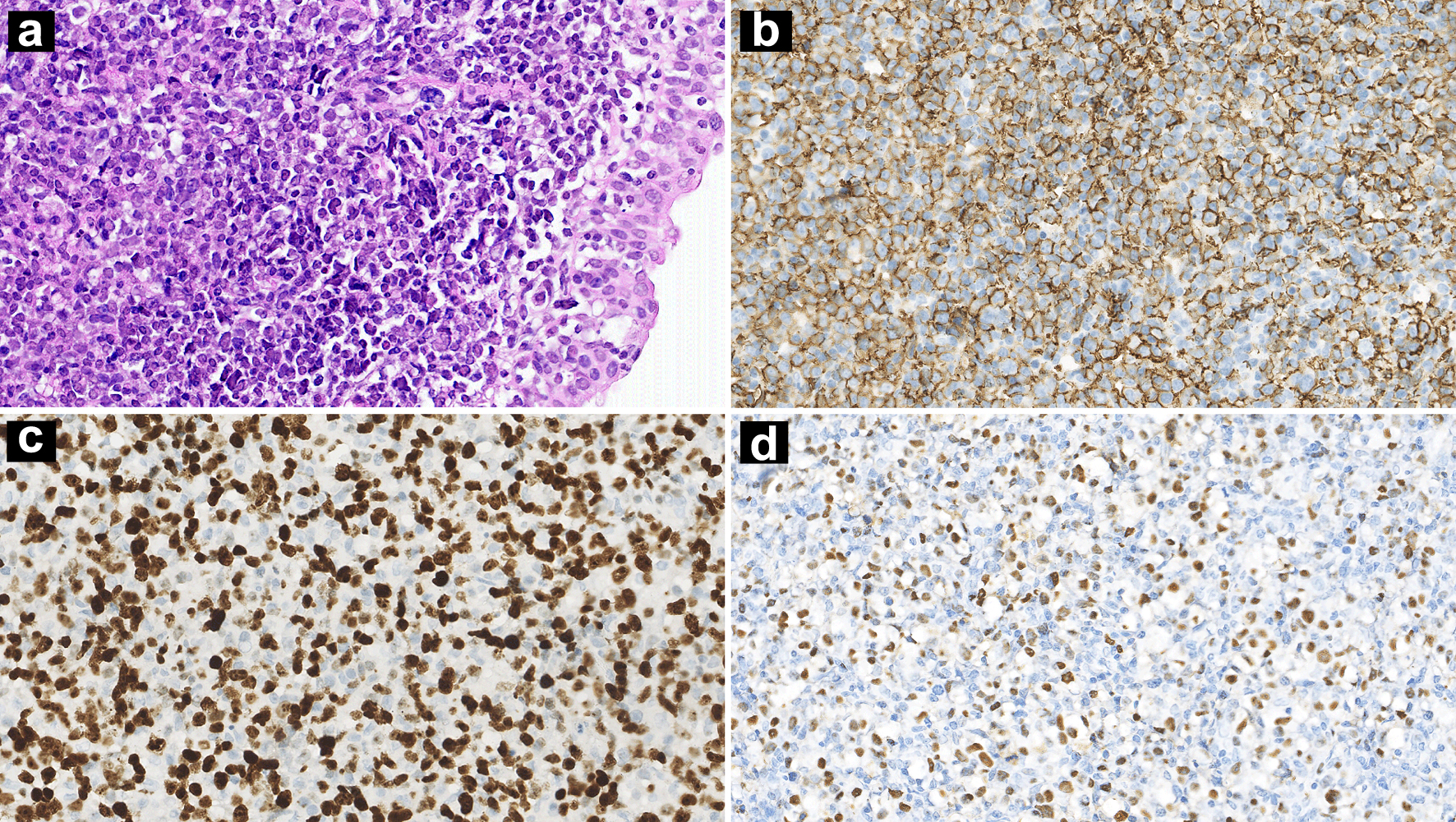 Click for large image | Figure 1. Nasopharynx biopsy showing diffuse infiltration of atypical lymphoid cells (a: H&E, × 100 magnification). IHC stains showing CD20 positivity (b: × 100 magnification) and a high Ki-67 index (c: × 100 magnification). ISH for EBERs was positive (d: × 100 magnification). H&E: hematoxylin and eosin; IHC: immunohistochemistry; ISH: in situ hybridization; EBER: Epstein-Barr virus-encoded small RNA. |
Bilateral cervical lymphadenopathy was confirmed by ultrasound, on which the larger lymph node on the right side was 35 × 18 mm, and another one sized 35 × 20 mm on the left. The spleen was enlarged with a thickness of about 5.2 cm. Excisional biopsy was performed on the left cervical lymph node. Histopathological examination showed that the tissue architecture of lymph nodes was totally effaced by medium-sized to large lymphocytes arranging in a diffuse pattern. Focal coagulative necrosis was also observed. These atypical lymphocytes expressed CD79a, CD20, BCL-6, MUM1, and CD30. Ki-67 index was about 80%. The positive rates of BCL-2 and C-MYC were about 80% and 30%, respectively. EBER was detected (Fig. 2). Clonal IGH amplification and low IGK amplification peaks were found, product sizes of which were the same as that of nasopharynx biopsy. Subsequently, translocations of BCL-2, BCL-6 or C-MYC were not spotted by fluorescence in situ hybridization (FISH) using break apart probes.
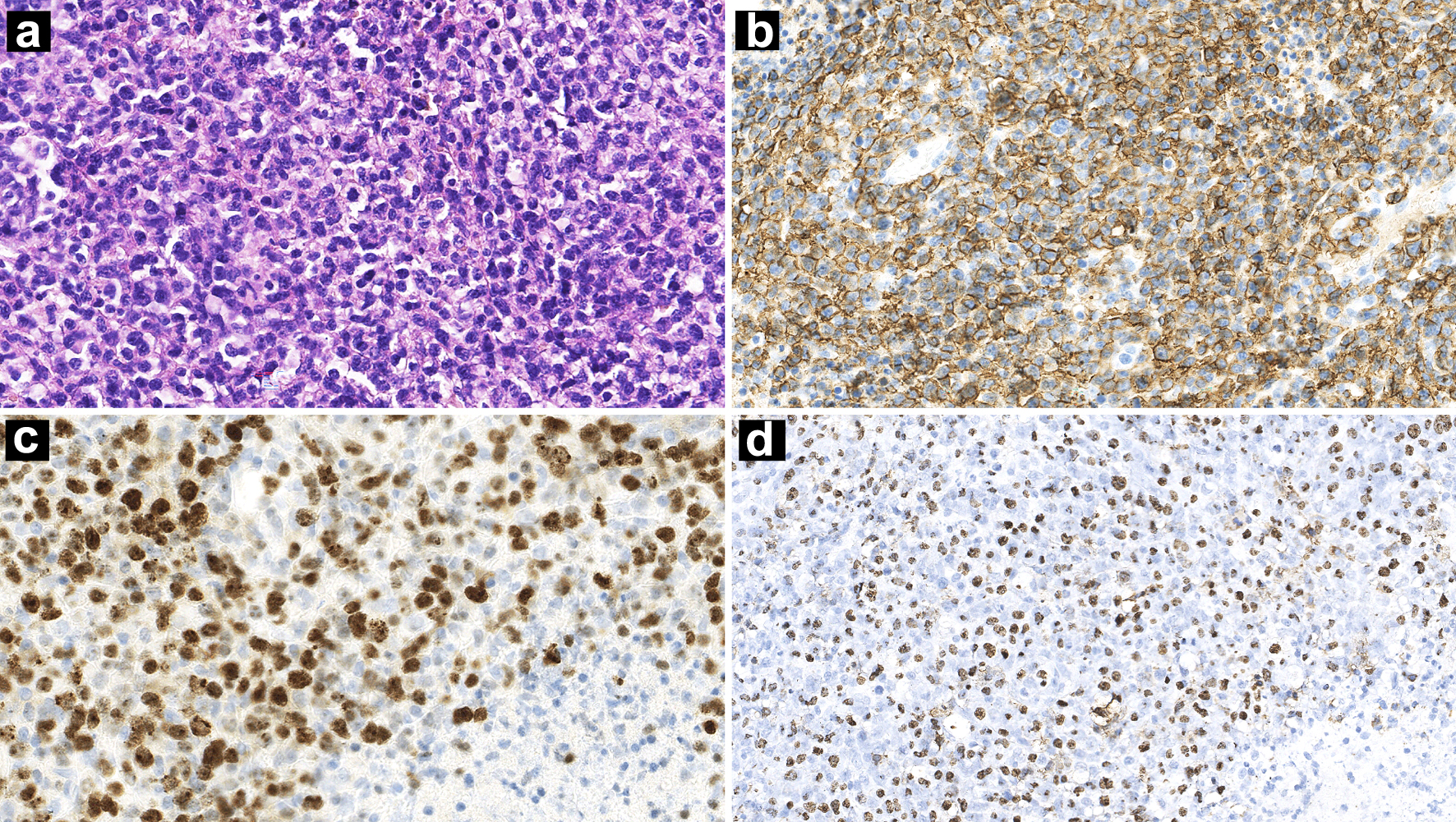 Click for large image | Figure 2. Lymph nodes biopsy showing diffuse proliferation of lymphoid cells accompanied by necrosis (a: H&E, × 100 magnification). IHC stains showing CD20 positivity (b: × 100 magnification) and a high Ki-67 index (c: × 100 magnification). ISH for EBERs was positive (d: × 100 magnification). H&E: hematoxylin and eosin; IHC: immunohistochemistry; ISH: in situ hybridization; EBER: Epstein-Barr virus-encoded small RNA. |
The patient’s fecal occult blood test was positive. Scattered mucosal swellings with erosion of varying sizes were found by colonoscopy. The largest lesion was about 2.5 × 3.0 cm localized in rectum (Fig. 3), and its mucosal biopsy showed diffuse infiltration of large atypical lymphocytes in laminae propria and submucosa. These atypical lymphocytes had the same immunophenotype as CD20, CD30 positivity, and a high Ki-67 index. EBER positivity, clonal IGH amplification, and low IGK amplification peaks with similar product sizes were found (Fig. 4).
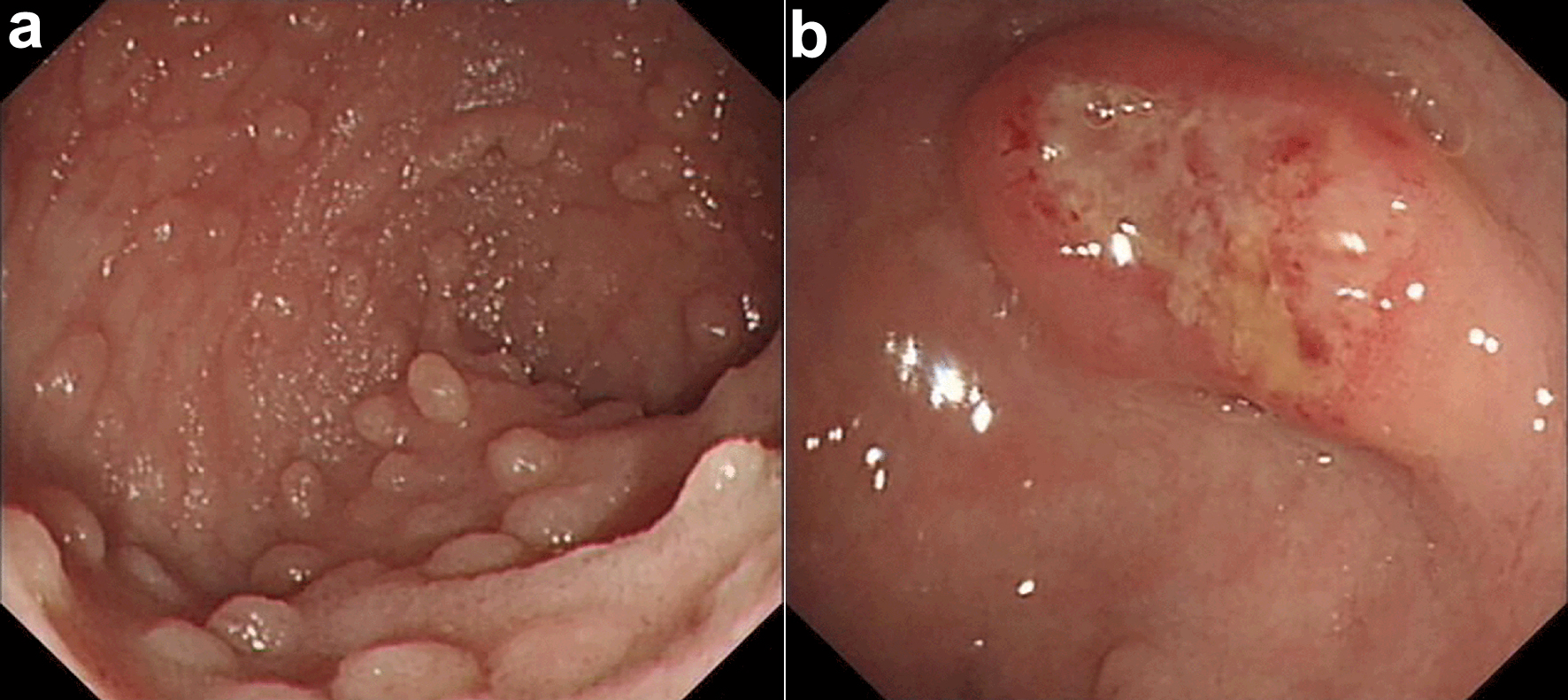 Click for large image | Figure 3. Endoscopic characteristic of case. Scattered mucosal swellings of varying sizes were found (a). The largest lesion was about 2.5 × 3.0 cm (b). |
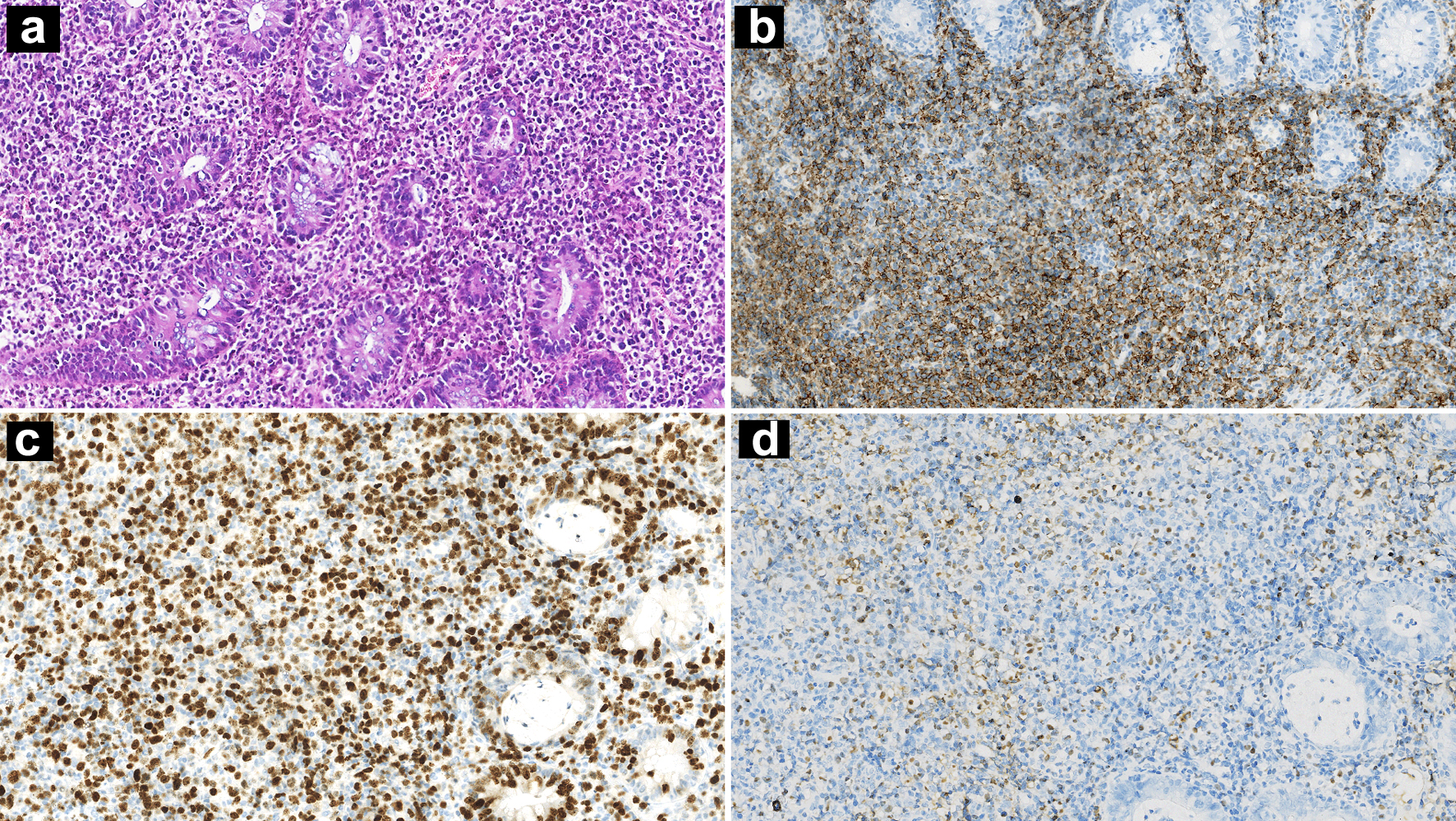 Click for large image | Figure 4. Intestinal mucosa biopsy showing diffuse infiltration of large atypical lymphocytes (a: H&E, × 100 magnification). IHC stains showing CD20 positivity (b: × 100 magnification) and high Ki-67 index (c: × 100 magnification). ISH for EBERs was positive (d: × 100 magnification). H&E: hematoxylin and eosin; IHC: immunohistochemistry; ISH: in situ hybridization; EBER: Epstein-Barr virus-encoded small RNA. |
No abnormal lymphocyte was detected by bone marrow aspiration and flow cytometry. Bone marrow tissue biopsy showed hypercellularity and immunostaining figured out few scattering small lymphocytes of CD20 positivity or CD3 positivity. Scattered EBER-positive signals were detected by ISH. However, it could not be identified whether the EBER-positive cells were B lymphocytes.
The diagnosis as EBV+ DLBCL was finally made. Positron emission tomography-computed tomography (PET-CT) scan showed that lymphoma involved cervical thoracoabdominal lymph nodes, pharynx, bilateral nasal cavity, spleen, subcutaneous and muscular soft tissue, gut, and left shoulder blade. The clinical stage was IVB.
The patient received four cycles of R-CHOP treatment. No active lesion was found by PET-CT as mid-term evaluation. Followed by the fifth cycle of R-CHOP, the patient had fever again. Complete blood cell count revealed pancytopenia (white blood cell (WBC) 0.42 × 109/L, hemoglobin (Hb) 112 g/L, platelet (Plt) 32 × 109/L). Lymphocyte proportion in peripheral blood was only 0.3%, while CD4+ T cells, CD8+ T cells, B cells, and natural killer (NK) cells count were as low as 0.001 × 109/L, 0.002 × 109/L, 0.000 × 109/L, and 0.0003 × 109/L, respectively. Ferritin was 18,239.2 ng/mL (reference: 30 - 400 ng/mL) and LDH was 1,685.2 IU/L (reference: 120 - 250 IU/L). Coagulation function, hepatic and renal biochemistry indexes were within normal range, except fibrinogen (1.68 g/L, reference: 2.0 - 4.0 g/L) and triglyceride (2.25 mmol/L, reference: 0.29 - 1.70 mmol/L). Plasma EBV-DNA quantification was less than 1,000 copies/mL. Mild splenomegaly was detected by CT scan. Bone marrow aspiration revealed hypercellularity and prominent hemophagocytosis. NK-cell activity, soluble CD25, and cytokine tumor necrosis factor (TNF)-α were within normal range. No pathogenic mutation in HLH-causative genes (AP3B1, BLOC1S6, CD27, IL2RG, ITK, LYST, MAGT1, PRF1, Rab27a, SH2D1A, STX11, STXBP2, TCN2, and UNC13D) was detected.
After treatment with VP-16, intravenous immunoglobulin and dexamethasone, the patient got complete remission (CR) evaluated by PET-CT. The patient underwent allogeneic hematopoietic stem cell transplantation (allo-HCT) mostly at urging of himself. On day +10, leukocytes and platelets were successfully transplanted. Repeated reexamination of chimerism after transplantation suggested full donor implantation. No fever occurred within 3 months after transplantation. Plasma EBV-DNA quantification was negative. Lymphocyte proportion in peripheral blood was only 19.5%, while CD4+ T cells, CD8+ T cells, B cells, and NK cells count elevated to 0.034 × 109/L, 0.026 × 109/L, 0.002 × 109/L, and 0.725 × 109/L, respectively. Unfortunately, disturbance of consciousness was observed after the patient fell down accidentally 3 months after the transplantation. Cranial CT showed bilateral frontal cerebral contusion. Though symptomatic treatment such as dehydration and cranial pressure reduction was taken, unconsciousness increased progressively. The patient died a month later.
| Discussion | ▴Top |
We present a uniquely rare EBV+ DLBCL in an immunocompetent young man, which systematically involved lymph nodes and extranodal sites. HLH was diagnosed during the chemotherapy. Treatments as combination chemotherapy, immunotherapy, and allo-HCT were taken. The patient got CR but died as a result of an accidental fall.
EBV was known as oncogenic virus. EBV-associated LPD consisted of a wide clinicopathological B, T, and NK cell-derived disease spectrum, from an inert process with spontaneous remission to an aggressive and fatal course [1]. IM is the delayed outcome of primary EBV infection in general and occurrences in children and young adults are typically seen, whose triad of characteristics are fever, pharyngitis, and lymphadenopathy [9, 10]. Foci of EBV-infected B immunoblasts can be found in the interfollicular regions of the tonsil inchoate IM [11]. Up to 50% of the memory B-cell population may be infected with EBV during IM, which allows EBV to persist for a long time. Significant expansions of atypically activated lymphocytes are the key feature of IM immune response [9]. Morphologically, B-immunoblastic proliferations along with reactive follicular hyperplasia are the characteristic feature. In some cases, due to excessive immunoblastic proliferation and architectural distortion, IM may be misdiagnosed as a lymphoma, notably DLBCL [12]. Differential diagnoses among IM, EBV-positive LPD associated with immunodeficiency, and EBV+ DLBCL are summarized in Table 1 [1, 13].
 Click to view | Table 1. Differential Diagnosis Among IM, EBV+ LPD with IDD, and EBV+ DLBCL |
Our patient was a young adult who presented with stinky runny nose and nasal discomfort, accompanied by fever and bilateral cervical lymphadenopathy. His initial biopsy was from nasopharynx revealing EBV-positive atypical B-cell proliferation. These clinicopathological manifestations implied IM as a significant differential diagnosis. Unfortunately, the patient also suffered from multiple intestinal ulcers. All biopsies from nasopharynx, lymph node, and intestinal mucosa verified pathological diagnosis as EBV+ DLBCL. It is understandable that making diagnosis of EBV+ DLBCL in the nasopharynx of young adults must be extremely cautious.
Defined as a large B-cell lymphoma where most tumor cells though lack of the history of immune deficiency or disorder (other than immune senescence) still harbor EBV, EBV+ DLBCL should accord with diagnostic criteria recommended by fifth WHO classification [2]. Our patient presented with nasopharynx mass, bilateral cervical lymphadenopathy, and multiple peptic ulcers. Pathological examination of these sites above proved clonal proliferation of large atypical B-lymphocytes with a high Ki-67 index. Both IGH and IGK gene rearrangements detected similar amplification peaks among these biopsies. Systemic EBV+ DLBCL in such immunocompetent young adults is rarely seen in the previous cases, especially involving digestive tract.
HLH is a multi-factorial hyperinflammatory syndrome, often caused by lymphoma or EBV infection. As HLH is liable to be a fatal disease, diagnosis and immunosuppressive therapy are urgently needed to control the hyperinflammation [14]. Unfortunately, our patient developed HLH during the treatment, meeting the HLH-2004 diagnostic criteria, bringing greater therapeutic challenges [15].
Although R-CHOP has improved survival outcomes in DLBCL, NOS patients, it induces higher response in EBV-negative DLBCL than in EBV+ DLBCL patients [16]. Autologous hematopoietic cell transplantation (auto-HCT) may be considered for patients with relapsed or refractory EBV+ DLBCL [17]. Allo-HCT is indicated for patients who have previously failed auto-HCT or could not undergo auto-HCT as the result of chemoresistance [18]. However, the exact sequence of hematopoietic cell transplantation (HCT) is still uncertain [19]. Some new approaches should be taken into account to address distinct pathways involved in EBV physiology and pathology, like viral replication, targeting EBV-specific activating pathways, and improvement of immune responses [16]. In a study of 10 patients with intractable EBV+ post-transplantation lymphoproliferative disease (PTLD) after allo-HCT, the use of γ-interferon capture to manufacture EBNA1-specific EBV-cytotoxic T lymphocytes (EBV-CTLs) took about 3 days and was capable of restoring T-cell immunity against EBV, which induced a 70% response rate [20]. Prospectively, targeting the programmed cell death ligand 1 (PD-L1)/programmed cell death 1 (PD-1) pathway hints a feasible treatment for EBV+ DLBCL. It is reported that using CD19 chimeric antigen receptor (CAR) T-cell therapy after PD-1 inhibition in a patient with intractable EBV+ DLBCL and HLH has succeeded [21].
Conclusion
EBV+ DLBCL is a highly aggressive lymphoma subtype. Initial nasopharynx biopsy is liable to be confused with IM and brings difficulty for making an accurate diagnosis, especially in young adults. Systemic disease involving both lymph nodes and extranodal sites and complicated by HLH is rarer but devastating. More similar cases prospectively should be collected. To better understand and meliorate the prognosis of this rare also challenging entity, a multi-dimension approach is urgently needed.
Learning points
EBV-associated B-cell LPD with a high Ki-67 index and IGH, IGK clonalities in nasopharynx occurring in young adults is quite challenging. The differential diagnosis should include IM and EBV+ DLBCL. Confirmation of nasopharyngeal EBV+ DLBCL demands systemic examination and proper biopsy. HLH can occur in time of lymphoma remission. Precision in treatment is crucial, accounting for potential interactions among therapies for lymphoma and complications such as HLH.
Acknowledgments
We would like to thank the clinicians, hematopathologists, radiologists, technicians and nurses for their efforts in diagnosing, treating the patient’s EBV+ DLBCL and HLH, and undertaking allo-HCT. We also thank the patient and his family for their support to this report.
Financial Disclosure
This case report has not been funded.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Informed consent was obtained from the patient’s family for the publication of this case report and any accompanying images.
Author Contributions
SYL collected and organized the data, and drafted the manuscript. SZ contributed to making pathological diagnosis and figures. GCH provided overall direction and guidance to the work. YW and GCH administered with the patient. SYL and GCH also provided critical revision for the manuscript. All authors approved the final version of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article. Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
allo-HCT: allogeneic hematopoietic stem cell transplantation; auto-HCT: autologous hematopoietic cell transplantation; CAR: chimeric antigen receptor; CHL: classic Hodgkin lymphoma; CNS: central nervous system; CR: complete remission; DLBCL: diffuse large B-cell lymphoma; EBER: Epstein-Barr virus-encoded small RNA; EBV: Epstein-Barr virus; EBV-CTLs: EBV-cytotoxic T lymphocytes; EBV+ DLBCL: EBV-positive diffuse large B-cell lymphoma; EBV+ LPDs: EBV-positive lymphoproliferative disorders; FISH: fluorescence in situ hybridization; GI: gastrointestinal; H&E: hematoxylin and eosin; HCT: hematopoietic cell transplantation; HLH: hemophagocytic lymphohistiocytosis; IHC: immunohistochemistry; IDD: immune deficiency/dysregulation; IM: infectious mononucleosis; ISH: in situ hybridization; LDH: lactate dehydrogenase; LPDs: lymphoproliferative disorders; LyG: lymphomatoid granulomatosis; PET-CT: positron emission tomography-computed tomography; PTLD: post-transplantation lymphoproliferative disease; R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone
| References | ▴Top |
- Quintanilla-Martinez L, Swerdlow SH, Tousseyn T, Barrionuevo C, Nakamura S, Jaffe ES. New concepts in EBV-associated B, T, and NK cell lymphoproliferative disorders. Virchows Arch. 2023;482(1):227-244.
doi pubmed pmc - Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, Bhagat G, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022;36(7):1720-1748.
doi pubmed pmc - Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019-5032.
doi pubmed pmc - Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390.
doi pubmed pmc - Hwang J, Suh CH, Won Kim K, Kim HS, Armand P, Huang RY, Guenette JP. The Incidence of Epstein-Barr Virus-Positive Diffuse Large B-Cell Lymphoma: A Systematic Review and Meta-Analysis. Cancers (Basel). 2021;13(8):1785.
doi pubmed pmc - Chinnici A, Beneforti L, Pegoraro F, Trambusti I, Tondo A, Favre C, Coniglio ML, et al. Approaching hemophagocytic lymphohistiocytosis. Front Immunol. 2023;14:1210041.
doi pubmed pmc - Kacar AG, Celkan TT. Hemophagocytic lymphohistiocytosis. Balkan Med J. 2022;39(5):309-317.
doi pubmed pmc - Han AR, Lee HR, Park BB, Hwang IG, Park S, Lee SC, Kim K, et al. Lymphoma-associated hemophagocytic syndrome: clinical features and treatment outcome. Ann Hematol. 2007;86(7):493-498.
doi pubmed - Williams H, Crawford DH. Epstein-Barr virus: the impact of scientific advances on clinical practice. Blood. 2006;107(3):862-869.
doi pubmed - Dunmire SK, Hogquist KA, Balfour HH. Infectious Mononucleosis. Curr Top Microbiol Immunol. 2015;390(Pt 1):211-240.
doi pubmed pmc - Anagnostopoulos I, Hummel M, Kreschel C, Stein H. Morphology, immunophenotype, and distribution of latently and/or productively Epstein-Barr virus-infected cells in acute infectious mononucleosis: implications for the interindividual infection route of Epstein-Barr virus. Blood. 1995;85(3):744-750.
pubmed - Rezk SA, Weiss LM. EBV-Associated Lymphoproliferative Disorders: Update in Classification. Surg Pathol Clin. 2019;12(3):745-770.
doi pubmed - Dojcinov SD, Quintanilla-Martinez L. How I diagnose EBV-positive B- and T-cell lymphoproliferative disorders. Am J Clin Pathol. 2023;159(1):14-33.
doi pubmed - Ponnatt TS, Lilley CM, Mirza KM. Hemophagocytic lymphohistiocytosis. Arch Pathol Lab Med. 2022;146(4):507-519.
doi pubmed - Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124-131.
doi pubmed - Malpica L, Marques-Piubelli ML, Beltran BE, Chavez JC, Miranda RN, Castillo JJ. EBV-positive diffuse large B-cell lymphoma, not otherwise specified: 2022 update on diagnosis, risk-stratification, and management. Am J Hematol. 2022;97(7):951-965.
doi pubmed - Bento L, Gutierrez A, Novelli S, Montoro J, Pinana JL, Lopez-Corral L, Cabrero M, et al. Allogeneic stem cell transplantation as a curative option in relapse/refractory diffuse large B cell lymphoma: Spanish multicenter GETH/GELTAMO study. Bone Marrow Transplant. 2021;56(8):1919-1928.
doi pubmed - Kanate AS, Majhail NS, Savani BN, Bredeson C, Champlin RE, Crawford S, Giralt SA, et al. Indications for hematopoietic cell transplantation and immune effector cell therapy: guidelines from the American society for transplantation and cellular therapy. Biol Blood Marrow Transplant. 2020;26(7):1247-1256.
doi pubmed - Hamadani M, Gopal AK, Pasquini M, Kim S, Qiu X, Ahmed S, Lazaryan A, et al. Allogeneic transplant and CAR-T therapy after autologous transplant failure in DLBCL: a noncomparative cohort analysis. Blood Adv. 2022;6(2):486-494.
doi pubmed pmc - Icheva V, Kayser S, Wolff D, Tuve S, Kyzirakos C, Bethge W, Greil J, et al. Adoptive transfer of epstein-barr virus (EBV) nuclear antigen 1-specific t cells as treatment for EBV reactivation and lymphoproliferative disorders after allogeneic stem-cell transplantation. J Clin Oncol. 2013;31(1):39-48.
doi pubmed - Chavez JC, Bachmeier C, Kharfan-Dabaja MA. CAR T-cell therapy for B-cell lymphomas: clinical trial results of available products. Ther Adv Hematol. 2019;10:2040620719841581.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


