| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 9, Number 3, March 2018, pages 86-89
Nuclear Protein of the Testis Midline Carcinoma
Ilda Coelhoa, e, Ana Antunesb, Catia Cabralc, Gloria Abreud, Guilherme Gomesa
aInternal Medicine Department, Braga Hospital, Portugal
bUrgency Department, Braga Hospital, Portugal
cInternal Medicine Department, Centro Hospitalar Tondela-Viseu, Portugal
dDepartment of Cardiology, Braga Hospital, Portugal
eCorresponding Author: Ilda Coelho, Internal Medicine Department, Braga Hospital, Portugal
Manuscript submitted January 6, 2018, accepted January 29, 2018
Short title: Nuclear Protein of Testis Midline Carcinoma
doi: https://doi.org/10.14740/jmc3001w
| Abstract | ▴Top |
Nuclear protein in testis (NUT) carcinomas are rare aggressive carcinomas characterized by chromosomal rearrangement that involve the gene encoding the NUT. This carcinoma was recognized in the thymus in 2004 WHO classification as a carcinoma with t(15;19) translocation, and it is also referred to as NUT carcinoma. However, currently, there is still sparse information about this entity which is treated as a very aggressive carcinoma with a median survival of 7 months. We report a case of a 20-year-old man with an aggressive growing pulmonary mass in the upper left lobe associated with bone metastasis. The bronchial and bones metastases biopsies presented a basaloid squamous cell carcinoma, which precipitated the examination for translocation t(15:19) and immunocytochemical study, both presenting positive results, which allowed establishing the diagnosis of NUT carcinoma.
Keywords: Nuclear protein in testis; Aggressive carcinomas; t(15;19) translocation
| Introduction | ▴Top |
Nuclear protein in testis (NUT) carcinomas are malignant epithelial neoplasias, characterized by chromosomal rearrangements in the gene encoding the NUT protein. It involves patients of different age groups, from newborns to 78 years of age. No significant difference in incidence according to gender or race, or association with any etiologic agent, was reported. Genetically, in NUT carcinomas, only a translocation is sufficient for the appearance of the lesion. Two-thirds of the cases are associated to t(15; 19) translocation, which conditions a fusion between the gene encoding the NUT protein and BRD4 (“bromodomain-containing protein 4”).
It is a rare entity, with few cases described in the literature, and about 63 cases were described [1].
The form of presentation is extremely variable with the location of the lesions, being caused mainly by the mass effect of the lesion. In the majority (66%) of the cases, they have already metastasized at the time of diagnosis. The definitive diagnosis is performed by karyotype analysis with demonstration of the translocation, or by identifying the fusion genes, through polymerase chain reaction (PCR) or in situ hydrolysis. However, these methods are not easily accessible in most hospital settings. The prognosis has been invariably fatal, with the mean survival time estimated at 6 - 7 months in most series, although some refer to 9 - 10 months.
| Case Report | ▴Top |
A 20-year-old man presented to the emergency department for a lower back pain, of sudden onset, worsened with movements, without irradiation and with pleuritic chest pain in the left hemithorax, which improved after administration of non-steroidal anti-inflammatory drugs (NSAIDs).
He was a smoker of 4 - 5 cigarettes per day, did not take any medications and denied alcohol or drugs abused.
In the family history, we highlight the fact that he had a brother who died at the age of 24, with a large mediastinal mass diagnosed a Ewing’s sarcoma.
On physical examination, the patient presented pain on mobilization and palpation of the left costal margin, lumbar spine and pelvis and, decreased of pulmonary sounds in the left hemithorax, without other auscultatory changes in the pulmonary parenchyma.
Serum analyses only revealed an increase of lactate dehydrogenase (475 U/L), being the rest of the study without renal dysfunction (urea 23 mg/dL, creatinine 0.9 mg/dL), without ionic alterations (potassium 3.8 mmol/L, sodium 144 mmol/L, corrected calcium 9.8 mg/dL, phosphorus 3.4 mg/dL), negative C-reactive protein and complete blood count within normal values. The human chorionic gonadotrophin hormone and alpha-fetoprotein were researched, which were both normal (> 1.0 mIU/mL and 2.07 ng/mL, respectively).
A large mediastinal hypotransparency on the left side (Fig. 1a) was revealed in the chest radiograph. For a better clarification of this image, a computed tomography (CT) of the chest revealed a pulmonary mass in the left upper pulmonary lobe, in contact with the aorta and the left pulmonary artery, mediastinal lymph nodes and bone metastases in the sternum and left costal arch (Fig. 1b). In the abdomen and pelvis, secondary lesions in the spine and iliac bone were observed in the CT scan (Fig. 1c, d).
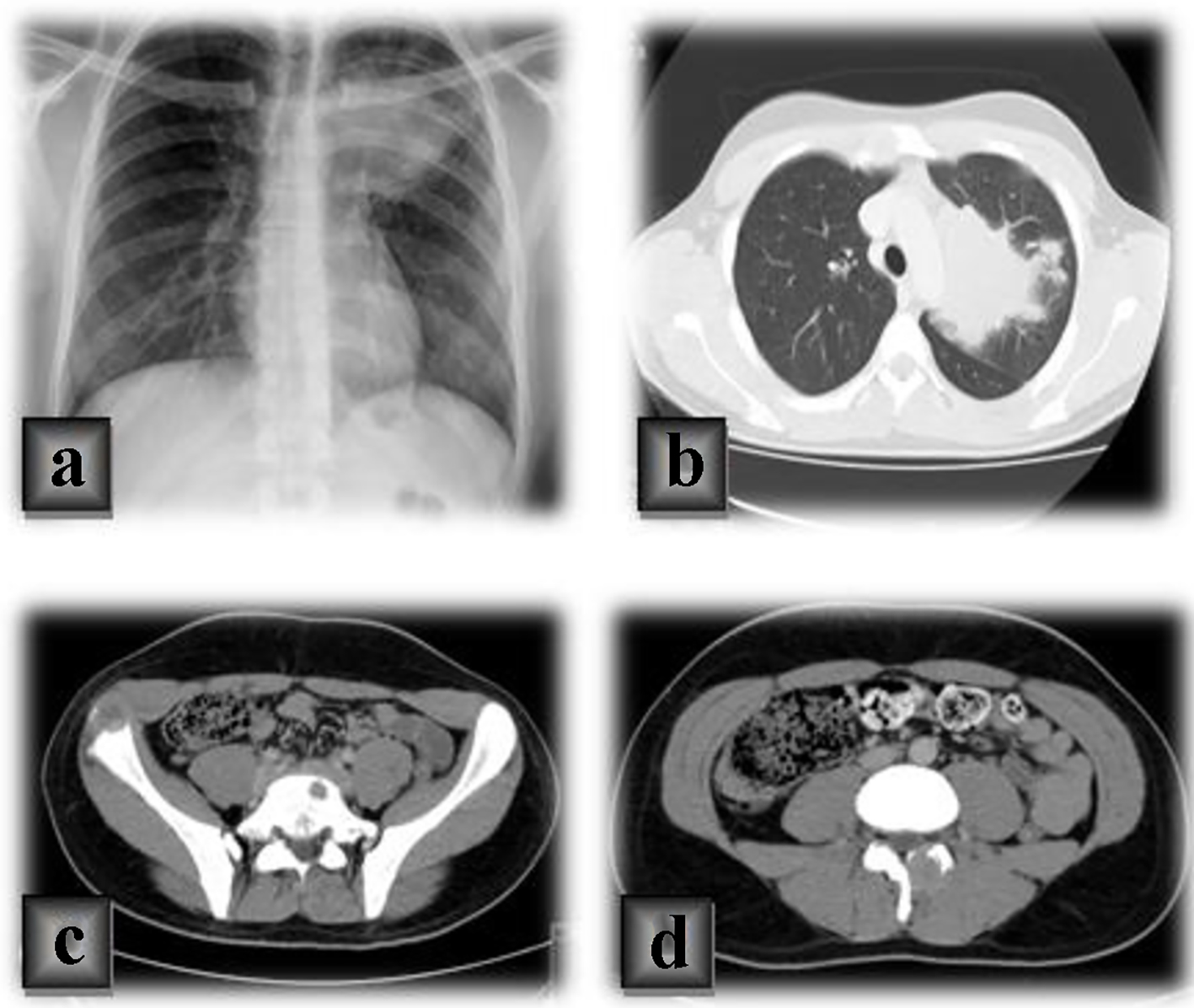 Click for large image | Figure 1. (a) Chest radiograph of a large mediastinal hypotransparency on the left side. (b) A chest computed tomography (CT) of a pulmonary mass in the left upper pulmonary lobe, in contact with the aorta and the left pulmonary artery, mediastinal lymph nodes and bone metastases in the sternum and left costal arch. (c, d) CT scan of secondary lesions in the spine and iliac bone in the abdomen and pelvis. |
The bone scintigraphy showed pathological foci in bilateral iliac bone, sternum, second and fifth left costal arches and the right lesser trochanter (Fig. 2).
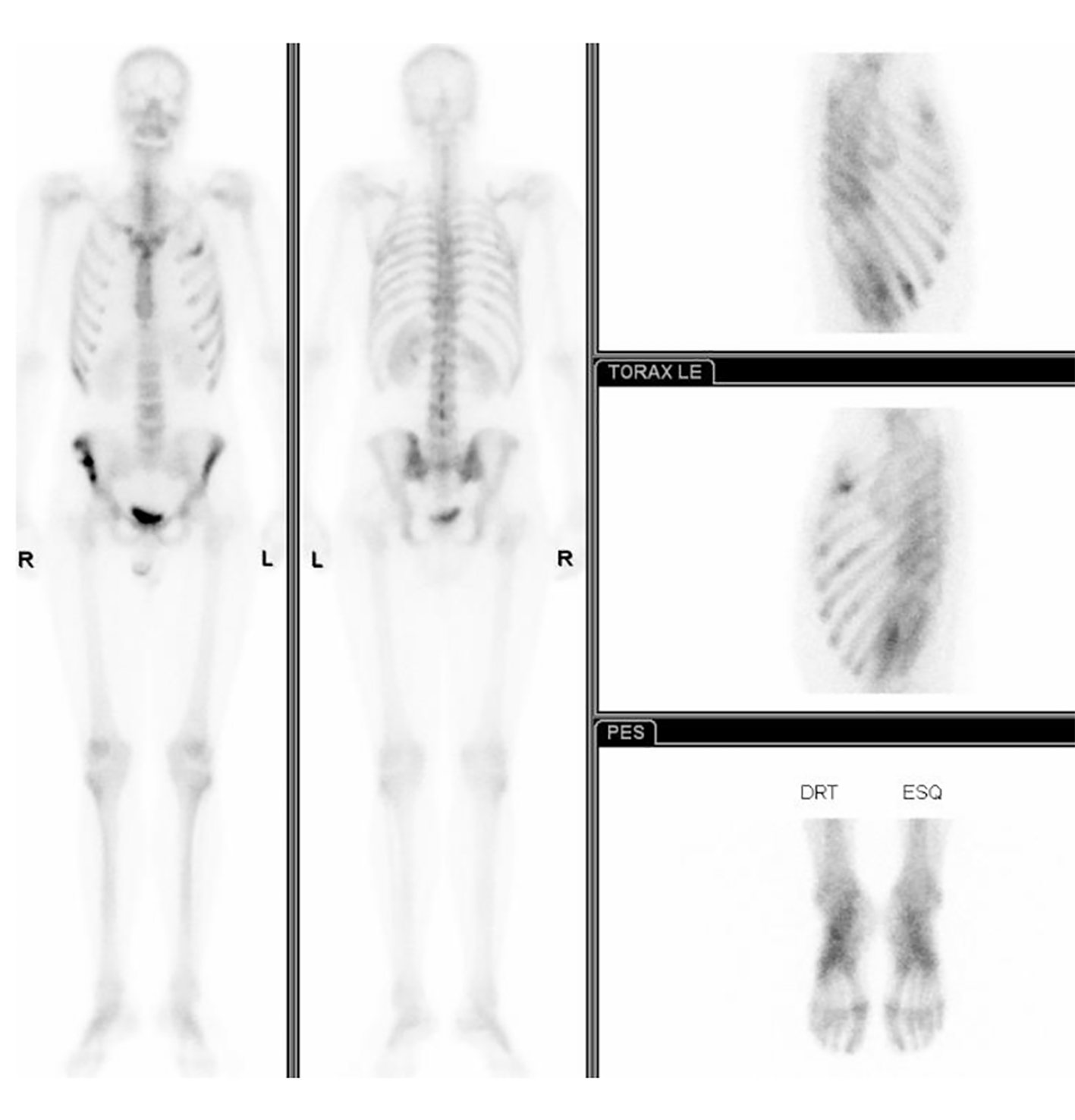 Click for large image | Figure 2. The bone scintigraphy showing pathological foci in bilateral iliac bone, sternum, second and fifth left costal arches and the right lesser trochanter. |
In the bronchofibroscopy, the upper division of the left lobar bronchus was total blocked by an endobronchial mass. Bronchial histological exam revealed a presence of ciliated cells of bronchial lining, alveolar macrophages, keratinized pavement cells and small cell stratified clusters, with poorly defined cytoplasm, poorly defined and rounded nuclei with fine chromatin and apparent small nucleoli. Due to the age of the patient and histological characteristics, an immunocytochemical study was performed, using anti-NUT monoclonal antibody which showed intense and diffuse nuclear positivity, thus demonstrating that this carcinoma was associated with translocation (15;19).
The histological examination of fragments of bone lesions of right iliac bone and sternal demonstrated dense fibrous tissue involved by the neoplasm with features that were overlapping with those identified in the histological examination of endobronchial tumor biopsy. The immunocytochemical study using the anti-NUT monoclonal antibody also demonstrated intense and diffuse nuclear positivity.
On the 10th day of hospitalization, the patient developed paralysis of the lower limbs associated with urinary retention in need of urinary catheter placement and uncontrolled pain. In a group decision, it was decided to perform palliative radiotherapy directed to D10 (site of spinal compression/destruction). After having completed 10 sessions of radiotherapy, improvement of the neurological and pain complaints was observed, with bladder sphincters recovery and an improvement in lower limb mobility.
After interdisciplinary discussions, the patient was submitted to a cycle of palliative chemotherapy with carboplatin and gemcitabine.
The outcome of this case was unfortunately fatal once the patient died 5 months after diagnosis.
| Discussion | ▴Top |
The term NUT midline carcinomas (NMCs) was used because this cancer is defined by rearrangement and translocation in the genes of NUT protein, and most of these tumors (about 90%) arise from midline anatomic sites [2, 3]. NMC is named for its tendency to involve midline structures [1].
The NUT carcinoma was recognized in the thymus in 2004 WHO classification as a carcinoma with t(15;19) translocation, and it is also referred to as NMC [4].
Although it was originally thought to be a disease of children and young adult, which is a reflection of the systematic research of neoplasms in young children, cases on new-borns to 78 years were described. Even so, most of those cases are found around the age of 15 years old, with 50% of the cases appearing over the age of 20 years old, and it seems to affect males and females equally [5].
A single translocation results in tumor transformation, classically characterized by the translocation involving chromosomes 15 and 19, t(15:19), in two-thirds of the cases, with the juxtaposition of BRD4 (19p13.1) and NUT (15q14) genes, that result in a BRD4-NTU fusion product [2, 6]. The fusion protein seems to block the epithelial and squamous differentiation.
Fewer than 63 of NUT carcinomas have been reported in the literature [1]. Several factors contribute to its apparent rarity: insufficient reporting, underdiagnosis, being a relatively new entity, age bias, and non-specific morphology.
The clinical symptoms are non-specific and include dyspnea, chronic coughing, nausea, pains from metastasis in bones and chest or shoulder pain [7]. They are also based in the location of the tumor and it is related mainly to the mass effect. At the time of diagnosis, metastases may present, with the bones being the most common site of extrathoracic involvement [7, 8].
There are no specific radiological signs, except for the suggestion of necrosis, confirmed in rare dissected specimens, which also show hemorrhagic areas. Histologically, it is composed of layers of small to medium cells, monotonous, with irregular nuclei and prominent nucleoli, and frequent images of mitosis and apoptosis. Squamous differentiation, cystic transformation and reaction with anti-keratin antibodies, mainly the CK7, often occur.
The differential diagnosis for NUT carcinoma includes undifferentiated carcinomas and poorly differentiated squamous cell carcinomas. It includes pediatric small cell tumors (primitive neuroectodermal tumor, rhabdomyosarcoma, desmoplastic small round cell tumor), melanoma, olfactory neuroblastoma, high-grade hematologic malignancies, endocrine carcinomas and other undifferentiated or poorly differentiated carcinomas. NUT carcinomas are monomorphic, poorly differentiated carcinomas with varying degrees of squamous differentiation, and they can mimic other undifferentiated neoplasms such as germ cell tumors, Ewing’s sarcoma or lymphoma.
The diagnosis is made by demonstrations of expression of the NUT-fusion protein using a monoclonal antibody to NUT for immunohistochemistry (sensitivity 87% and specificity and positive predictive value 100%), and confirmation of the fusion (BRD-NUT or NUT-variant) by fluorescent in situ hybridization or reverse transcriptase-polymerase chain reaction [6].
Patients have been receiving chemotherapy, in different combinations, without success, assuming natural chemoresistance. The radiotherapy has a palliative role and, more rarely, a neoadjuvant one, due to its low resectability.
In this patient, an urgent radiotherapy was used due to the compression of the spinal cord, continuing with palliative chemotherapy.
Currently, there is no defined therapy for this entity, in addition, durante to the aggressiveness and rapid tumor growth, surgical removal is not feasible. Apparently, the best option seems to be the combination of gemcitabine, docetaxel and cisplatin. The administration of suberoylanilide hydroxamic acid and extra-terminal proteins inhibitors is promising but further data are required [9].
The outcome has been invariably fatal despite the promising target therapies currently tested in clinical trial. The average survival rate still is between 6 and 7 months.
Grant Support
None.
Conflict of Interest
None.
| References | ▴Top |
- Sayapina MS, Savelov NA, Karseladze AI, Bulanov AA, Tryakin AA, Nosov DA, Garin AM, et al. Nuclear protein of the testis midline carcinoma masquerading as a primary mediastinal seminoma. Rare Tumors. 2016;8(2):6241.
doi pubmed - French CA. Pathogenesis of NUT midline carcinoma. Annu Rev Pathol. 2012;7:247-265.
doi pubmed - Teo M, Crotty P, O’Sullivan M, French CA, Walshe JM. NUT midline carcinoma in a young woman. J Clin Oncol. 2011;29(12):e336-339.
doi pubmed - Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243-1260.
doi pubmed - French CA. The importance of diagnosing NUT midline carcinoma. Head Neck Pathol. 2013;7(1):11-16.
doi pubmed - French CA. NUT midline carcinoma. Cancer Genet Cytogenet. 2010;203(1):16-20.
doi pubmed - Sholl LM, Nishino M, Pokharel S, Mino-Kenudson M, French CA, Janne PA, Lathan C. Primary pulmonary NUT midline carcinoma: clinical, radiographic, and pathologic characterizations. J Thorac Oncol. 2015;10(6):951-959.
doi pubmed - Stelow EB. A review of NUT midline carcinoma. Head Neck Pathol. 2011;5(1):31-35.
doi pubmed - Ueki H, Maeda N, Sekimizu M, Yamashita Y, Moritani S, Horibe K. A case of NUT midline carcinoma with complete response to gemcitabine following cisplatin and docetaxel. J Pediatr Hematol Oncol. 2014;36(8):e476-480.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


