| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 6, June 2022, pages 253-256
Iatrogenic Thoracic Duct Injury via the Right Internal Jugular Vein: A Case Report
Hiroki Konoa, Daiki Shakoa, Yoshimi Satoa, Tatsuya Kawasakia, b
aDepartment of Cardiology, Matsushita Memorial Hospital, Moriguchi, Osaka, Japan
bCorresponding Author: Tatsuya Kawasaki, Department of Cardiology, Matsushita Memorial Hospital, Sotojima 5-55, Moriguchi, Osaka 570-8540, Japan
Manuscript submitted October 18, 2021, accepted November 16, 2021, published online June 11, 2022
Short title: Thoracic Duct Injury via Right Jugular Vein
doi: https://doi.org/10.14740/jmc3811
| Abstract | ▴Top |
Thoracic duct injury is a rare mechanical complication during the insertion of a central venous cannula via the left internal jugular vein. We report a case of thoracic duct injury during the insertion of a temporary pacing lead via the right internal jugular vein. A 92-year-old woman presented with third-degree atrioventricular block. Temporary ventricular pacing was attempted via the right internal jugular venous route, but a guidewire and sheath migrated into the vessel structure that was not directly connected to the right ventricle. Considering the characteristics of the fluid obtained from the vessel and the anatomical components of the mediastinum, a diagnosis of thoracic duct injury was made. The system inserted incorrectly was removed and a pacing lead was placed in the right ventricular apex through the right internal jugular vein. Her clinical course was uneventful without developing pneumothorax, hemothorax, or chylothorax, and 5 days later, a permanent pacemaker was implanted via the left subclavian venous route.
Keywords: Central venous cannulation; Complication; Right internal jugular vein; Thoracic duct
| Introduction | ▴Top |
Central venous cannulation is an essential procedure in various medical fields such as for hemodynamic measurement, cardiac pacing, and nutritional support. The common mechanical complications include arterial puncture, hematoma, hemothorax, and pneumothorax [1, 2]. Thoracic duct injury is also known as a rare mechanical complication during the insertion of a central venous catheter via the left internal jugular vein because of its anatomical features [3]. Attention should be paid to the variants of the thoracic duct since complete right-sided thoracic duct is reported to be recognized as normal variants [4]. We report a case of thoracic duct injury during the insertion of a temporary pacing lead via the right internal jugular vein.
| Case Report | ▴Top |
Investigations
A 92-year-old woman was transferred to the emergency room of our hospital because of exertional general fatigue. The patient was in a normal state of health until 2 days before presentation, when her family member noticed that she had difficulty moving, followed by decreased oral intake. She had no dyspnea at rest or chest pain. Reviews of system were negative for weight loss, night sweat, coughs, muscle pains, abdominal pains, nausea, vomiting, and headaches. Her previous medical history was notable for traumatic intracerebral hemorrhage 2 months earlier, transcatheter aortic valve implantation for severe aortic stenosis 4 months earlier, and subarachnoid hemorrhage approximately 50 years earlier. Medications included rosuvastatin at a dose of 2.5 mg daily, clopidogrel sulfate at a dose of 50 mg daily, esomeprazole magnesium hydrate at a dose of 20 mg daily, epinastine at a dose of 10 mg daily, sodium ferrous citrate at a dose of 50 mg daily, and alendronate sodium hydrate at a dose of 35 mg weekly. The patient did not smoke or drink, and had no known allergies except for a possible allergy to antibiotics more than 20 years earlier.
She was alert and appeared to be in distress. On examination, the blood pressure was 208/79 mm Hg, pulse was 50 beats per minute, body temperature was 37.4 °C, and oxygen saturation was 96% while breathing ambient air. The jugular venous pressure was not high and a grade 2 diastolic murmur was audible with no gallops. No pulmonary rales were heard and there was no edema in the legs.
Electrocardiography demonstrated third-degree atrioventricular block with a ventricular rate of 46 beats per minute, a normal axis, a QRS duration of 106 ms, no ST-T segment changes, and left ventricular high voltage. Chest radiography revealed cardiomegaly and pulmonary congestion without pleural effusion. Bedside echocardiography demonstrated no notable findings except for an artificial aortic valve and moderate aortic regurgitation, findings unchanged from those obtained three months earlier. The complete blood cell counts, the levels of renal and liver function tests, and electrolyte balance were unchanged from those obtained approximately 1 month earlier, but the level of brain natriuretic peptide increased from 210.6 to 1,553.3 pg/mL (reference value, ≤ 18.4).
Temporary ventricular pacing was planned after receiving informed consent from the patient. Under maximal sterile-barrier precautions, a guidewire was inserted through a 20-gauge needle, after the first attempt with the landmark-based technique instead of ultrasound guidance, via the right internal jugular vein using the Seldinger technique in the supine position with the head rotated slightly away from the site of cannulation (Fig. 1a). A 6.0-Fr sheath was then proceeded into the superior vena cava, but the attempt was halted because of the patient’s discomfort and resistance to the insertion. No liquid reflux (i.e., blood) was withdrawn through the sheath, which was approximately 10 cm in length, even though it was inserted halfway. The sheath was expected to be in the vascular system based on the movement of the guidewire along the stable route, but the vessel was not likely to be directly connected to the heart (Fig. 1b). After a 5-mL saline flush into the vessel without resistance, cloudy, yellow liquid was retrieved (Fig. 2). Injection of contrast material through the sheath was not performed to avoid additional complications. The obtained fluid had a protein level of 915 mg/dL, blood sugar level of 70 mg/dL, and specific gravity of 1.008. Further examination of the liquid was not performed because of sample damage.
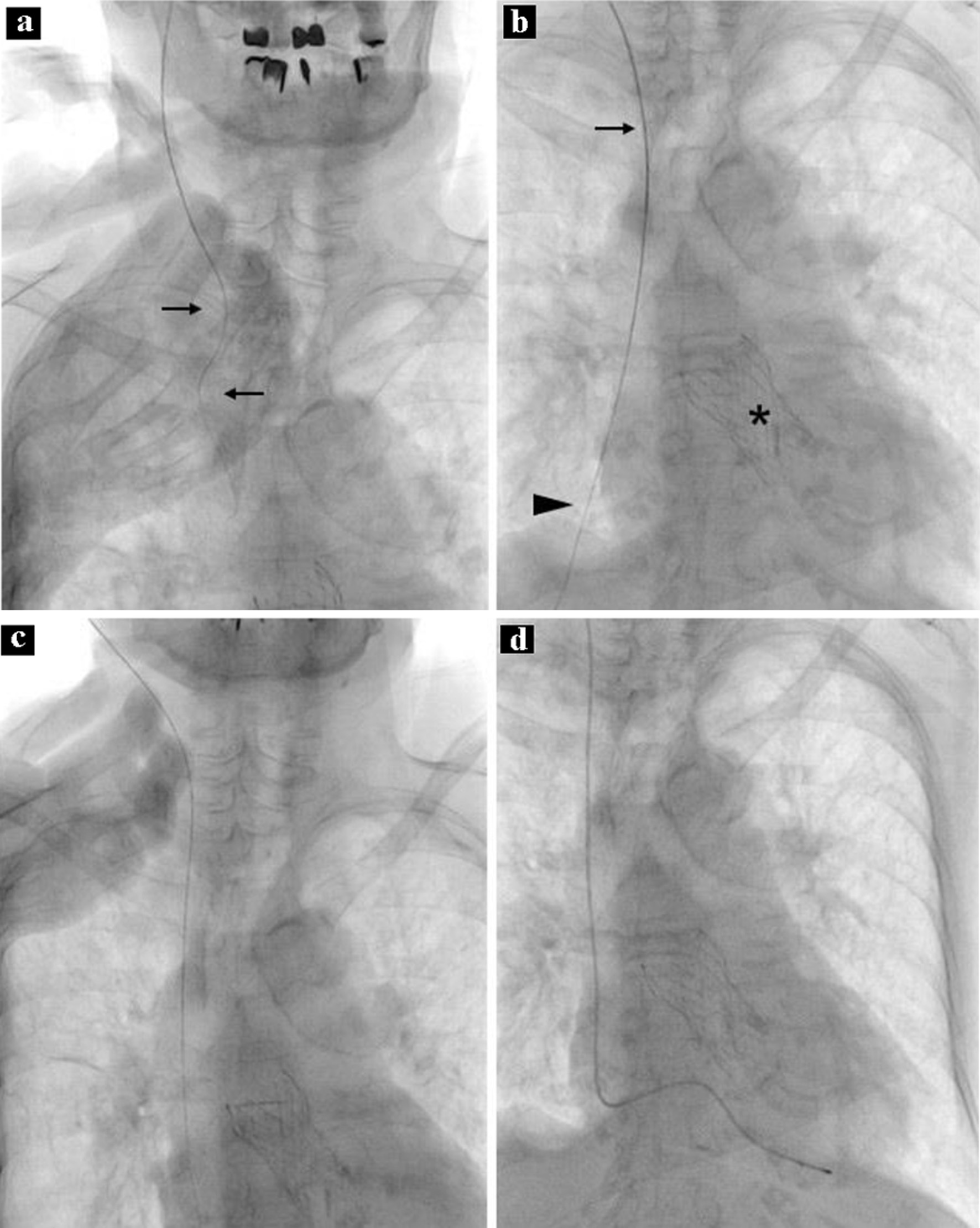 Click for large image | Figure 1. Procedure during temporary pacing. Note that the wire was not straight (a, arrows). A sheath was proceeded along the guidewire; the top of the sheath is in the upper part of the superior vena cava (b, arrow). Note that the distal part of the guidewire was not in the shadow of the heart (b, arrowhead). The asterisk shows an artificial aortic valve. After removal of the sheath and guidewire, the guidewire is correctly inserted into the superior vena cava, right atrium, and inferior vena cava (c). A temporary pacing lead is placed at the apex of the right ventricle (d). |
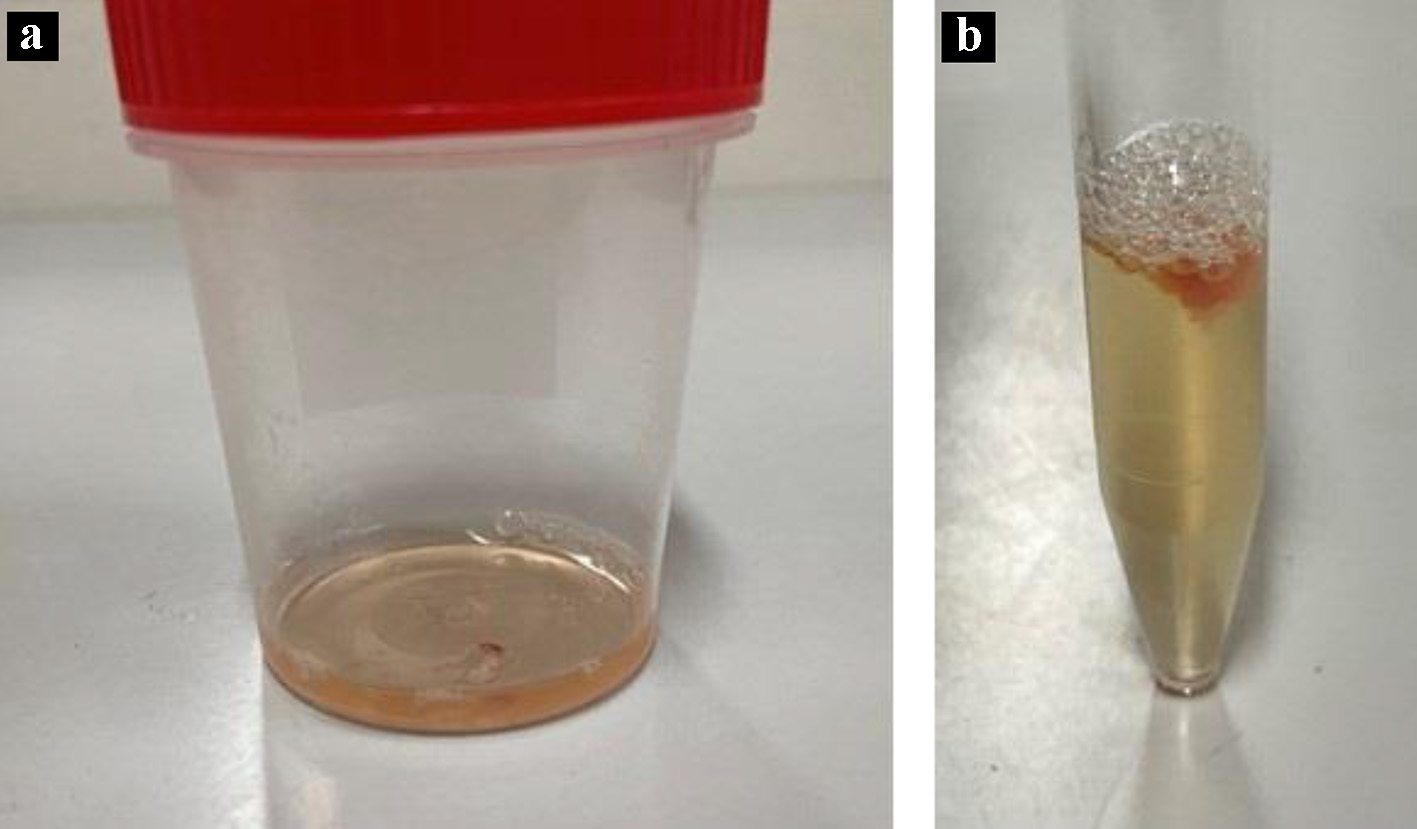 Click for large image | Figure 2. Liquid appearance. The liquid is cloudy and yellow (a); fibrin formation is suspected on the top of the liquid (b). |
Diagnosis
A presumed diagnosis of thoracic duct injury was made, given the anatomy of the mediastinum.
Treatment
The sheath and guidewire were removed. The patient was asymptomatic and her vital signs were stable without any specific procedures such as compression of the puncture site, oxygen supplementation, or Trendelenburg’s position. The sheath was inserted into the superior vena cava through the second attempt of the Seldinger technique after confirming that the guidewire was in the correct position (Fig. 1c). A temporary pacing lead was placed in the apex of the right ventricle without difficulty (Fig. 1d). No congenital abnormality, e.g., situs inversus, or harmful consequences associated with the first attempt, e.g., pneumothorax or hemothorax, were noted on computed tomography performed immediately after the temporary pacing lead insertion, although the thoracic duct was difficult to identify. No evidence of fluid retention was noted in the upper mediastinum.
Follow-up and outcomes
Her clinical course was uneventful without developing pneumothorax, hemothorax, or chylothorax. Five days later, permanent pacemaker implantation was performed.
| Discussion | ▴Top |
We report a case of third-degree atrioventricular block in which temporary cardiac pacing was performed. Central venous cannulation was attempted via the right internal jugular venous route, but a guidewire and sheath migrated into the vessel structure that was not directly connected to the right ventricle. Considering the characteristics of the fluid obtained from the vessel and the anatomical components of the mediastinum, it was prudent to make a diagnosis of thoracic duct injury in the current case, although the source of the fluid remained to be elucideated. The system inserted in the thoracic duct was removed and a pacing lead was placed in the right ventricular apex through the right internal jugular vein.
Procedures for central venous cannulation always carry risks of complications with an overall incidence of around 15%: mechanical complications in 5-19% of patients, infectious complications in 5-26%, and thrombotic complications in 2-26% [1, 2]. Although multiple sites are available for central venous cannulation, such as the internal jugular vein, subclavian vein, and femoral veins, the internal jugular or subclavian venous route is recommended, unless contraindicated, because the femoral venous route is more likely to be associated with mechanical complications (i.e., the frequency, 12.8-19.4%) [1]. The internal jugular approach and subclavian approach have a similar overall incidence of mechanical complications (the frequency,6.3-11.8% vs. 6.2-10.7%, respectively), although arterial puncture is more common in the jugular approach, whereas the subclavian approach carries more risks of pneumothorax and hemothorax [1].
Based on the anatomical location of the thoracic duct, it is reasonable to consider that it can be injured during central venous cannulation, albeit considered markedly rare. The thoracic duct usually originates from the cisterna chyli at T12 to L2 to the right of the aorta, courses cranially between the aorta and azygos vein, crosses the midline to the left at approximately the T5 to T6 vertebral levels, runs behind the internal jugular vein in the superior mediastinum, and drains into the venous system at the junction of the left internal jugular and subclavian veins [4]. This is why thoracic duct injuries are associated with the use of the left internal jugular vein [3]; the needle can puncture the orifice of the thoracic duct into the left brachiocephalic vein [5]. Thoracic duct injury developed in the current patient, although the right internal jugular approach was used to insert a temporary pacing lead. Of note, there are significant anatomic variants of the thoracic duct; complete right-sided thoracic duct and cisterna chyli emptying into the right venous angle, without crossing the midline, should be recognized as normal variants [4]. Thus, injuries of the thoracic duct or right lymphatic duct have been reported during right-sided venous catheterization [6, 7].
As the thoracic duct drains upwards of 75% of lymphatic fluid throughout the body and carries 1 - 2 L of lymphatic fluid per day [8], its injury may result in fluid retention in the mediastinum or chronic chylothorax after removal of the central venous line [5]. Considering the unacceptable mortality rate due to plasma protein loss or respiratory function impairment associated with thoracic duct injury [4, 9], early recognition of thoracic duct injury is important, although difficult because of its rarity and differing appearance of lymphatic fluids such as clear, straw-colored, or milky white [10]. Conservative management with dietary restriction is acceptable in patients with a chylous output of 1 L or less, but embolization or surgical ligation of the thoracic duct is recommended if the output is more than 1 L per day [4, 11, 12]. In the current patient, no treatment was required for the thoracic duct injury.
In conclusion, our case highlights the importance of acknowledging that thoracic duct injury can develop even via the right internal jugular approach for a central venous catheter.
Learning objective
Thoracic duct injury is a rare mechanical complication during central venous cannulation via the left internal jugular vein. We report a case of thoracic duct injury during the insertion of a temporary pacing lead via the right internal jugular vein.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained.
Author Contributions
HK, DS, YS, and TK contributed to data analysis and study design; HK and TK wrote the paper; DS and YS reviewed the paper; all authors gave the final approval.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003;348(12):1123-1133.
doi pubmed - Eisen LA, Narasimhan M, Berger JS, Mayo PH, Rosen MJ, Schneider RF. Mechanical complications of central venous catheters. J Intensive Care Med. 2006;21(1):40-46.
doi pubmed - Kwon SS, Falk A, Mitty HA. Thoracic duct injury associated with left internal jugular vein catheterization: anatomic considerations. J Vasc Interv Radiol. 2002;13(3):337-339.
doi - Johnson OW, Chick JF, Chauhan NR, Fairchild AH, Fan CM, Stecker MS, Killoran TP, et al. The thoracic duct: clinical importance, anatomic variation, imaging, and embolization. Eur Radiol. 2016;26(8):2482-2493.
doi pubmed - Teichgraber UK, Nibbe L, Gebauer B, Wagner HJ. Inadvertent puncture of the thoracic duct during attempted central venous catheter placement. Cardiovasc Intervent Radiol. 2003;26(6):569-571.
doi pubmed - Arditis J, Giala M, Anagnostidou A. Accidental puncture of the right lymphatic duct during pulmonary artery catheterization. A case report. Acta Anaesthesiol Scand. 1988;32(1):67-68.
doi pubmed - Ryan DW. Lymph leakage following catheterization of the right subclavian vein. Anesth Analg. 1978;57(1):123-124.
doi pubmed - Skandalakis JE, Skandalakis LJ, Skandalakis PN. Anatomy of the lymphatics. Surg Oncol Clin N Am. 2007;16(1):1-16.
doi pubmed - Cope C. Management of chylothorax via percutaneous embolization. Curr Opin Pulm Med. 2004;10(4):311-314.
doi pubmed - Lv S, Wang Q, Zhao W, Han L, Wang Q, Batchu N, Ulain Q, et al. A review of the postoperative lymphatic leakage. Oncotarget. 2017;8(40):69062-69075.
doi pubmed - Marts BC, Naunheim KS, Fiore AC, Pennington DG. Conservative versus surgical management of chylothorax. Am J Surg. 1992;164(5):532-534; discussion 534-535.
doi - Thompson KJ, Kernstine KH, Grannis FW, Jr., Mojica P, Falabella A. Treatment of chylothorax by robotic thoracic duct ligation. Ann Thorac Surg. 2008;85(1):334-336.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


