| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 6, June 2022, pages 257-262
Esophageal Pancreatic Acinar Heterotopia: Premalignant or Benign
Krystal Millsa, c , Temitayo Gboluajea, Timothy Sobukonlaa, Melvin Simienb
aDepartment of Internal Medicine, Morehouse School of Medicine, Atlanta, GA 30310, USA
bDepartment of Gastroenterology, Morehouse School of Medicine, Atlanta, GA 30310, USA
cCorresponding Author: Krystal Mills, Department of Internal Medicine, Morehouse School of Medicine, Atlanta, GA 30310, USA
Manuscript submitted December 6, 2021, accepted May 19, 2022, published online June 11, 2022
Short title: Esophageal Pancreatic Acinar Heterotopia
doi: https://doi.org/10.14740/jmc3838
| Abstract | ▴Top |
Dysphagia, which refers to difficulty swallowing, can be caused by benign pathologies of the esophagus such as gastroesophageal reflux disease which is the most common cause. There are also malignant pathologies such as esophageal carcinoma which should be excluded during the initial clinical evaluation of a patient. Esophageal pancreatic acinar heterotopia (EPAH) is an exceedingly rare finding and an uncommon differential for dysphagia. A search of the literature yielded few previously reported cases. In general, the reported prevalence of pancreatic acinar heterotopia ranges from 16% to 24% in asymptomatic patients and 3% in patients with a known history of Barrett’s esophagitis. It has been found in patients ranging from as young as 1 day old to an incidental autopsy finding. Here, we present a brief literature review and a case of a 57-year-old man with severe dysphagia who was discovered to have EPAH in the gastroesophageal junction, associated with active inflammation and focal metaplasia.
Keywords: Pancreatic acinar heterotopia; Esophageal; Dysphagia
| Introduction | ▴Top |
Esophageal pancreatic acinar heterotopia (EPAH) is an uncommon cause of dysphagia [1-7], and this entity is rarely reported in the literature [8-11]. The heterotopic pancreas lacks any continuity with the main body of the orthotopic pancreas [8, 9, 11]. Pancreatic acinar heterotopia is unusual in the esophagus, but has been more commonly described in other areas of the gastrointestinal tract such as the stomach, duodenum, jejunum, and ileum [8, 11, 12]. In general, patients with pancreatic acinar heterotopia are asymptomatic and as a result, most go undiagnosed. They have been discovered in 0.5-13.7% of autopsies [13, 14], and 0.2-0.5% of laparotomies [13]. For the cases in which patients develop symptoms such as dysphagia or abdominal pain, these symptoms are due to the mass effect and relative location of heterotopia to esophageal mucosa.
In any patient who presents with dysphagia and concomitant alarming symptoms similar to this index case, guidelines recommend the use of upper gastrointestinal endoscopy in the initial evaluation. This has diagnostic benefits such as distinguishing between a benign or non-benign cause, as well as therapeutic benefits through interventions such as esophageal dilation. In this case report, we present a 57-year-old man who was diagnosed with EPAH, after esophagogastroduodenoscopy (EGD) with biopsy, revealing the challenge of the symptom presentation and the appropriate diagnostic workup and management thereafter.
| Case Report | ▴Top |
Investigations
The patient was a 57-year-old man with known medical history of degenerative joint disease (DJD), coronary artery disease (CAD), hypertension, gastroesophageal reflux disease and chronic constipation who presented to the gastroenterology clinic for evaluation of difficulty swallowing. The patient reported a progressive worsening of this symptom over the preceding 4 months. He reported dysphagia to solids, further described as a sensation of food being stuck in his throat. He denied dysphagia to liquids. He endorsed associated abdominal pain characterized as an occasional burning sensation that was localized to the epigastric region. He also experienced 30-pound unintentional weight loss during the 4 months since onset of symptoms. He denied nausea, vomiting, hematemesis, odynophagia, hematochezia or melena.
He had history of a colonoscopy 1 year prior, which documented findings of internal and external non-thrombosed hemorrhoids, as well as a single tubular adenoma. He had a significant family history of colon cancer. Social history was significant for a 10-pack-year tobacco smoking history and alcohol use socially. His current medications included hydrochlorothiazide, amlodipine, lisinopril, gabapentin, acetaminophen, naproxen, cyclobenzaprine, atorvastatin and esomeprazole. He had no known allergies.
On presentation to the gastroenterology clinic, he had a body mass index (BMI) of 30.92 kg/m2 and his blood pressure was 149/75 mm Hg, with other vital signs within normal limits. His physical examination revealed no acute abnormalities for his general, neurological, cardiopulmonary or musculoskeletal systems. In regards to his abdominal examination, he had benign findings including normoactive bowels sounds and a non-tender abdomen.
Diagnosis
His laboratory tests from a month prior to his gastroenterology clinic were reviewed. These included hemoglobin of 14.7 g/dL, white blood cell of 6.4 × 103/µL, platelet of 160 × 103/µL, sodium of 138 mEq/L, potassium of 4.3 mEq/L, creatinine of 1.3 mg/dL, and blood urea nitrogen of 7 mg/dL. All other values for hematology and metabolic panel were within normal limits.
Given the presence of alarming features associated with our patient’s dysphagia on this initial presentation, he was scheduled for an elective upper gastrointestinal endoscopy in 2 weeks. He was also prescribed polyethylene glycol and psyllium for constipation.
Treatment
Follow-up EGD was completed 2 weeks after the clinic visit, and was significant for findings of an approximately 5 × 3 cm raised pink rubbery mass with overlying irregular mucosa at the gastroesophageal (GE) junction. This was partially resected for biopsy (Figs. 1 and 2). There was also irregular gastric mucosa visualized during the EGD and additional biopsy samples were taken.
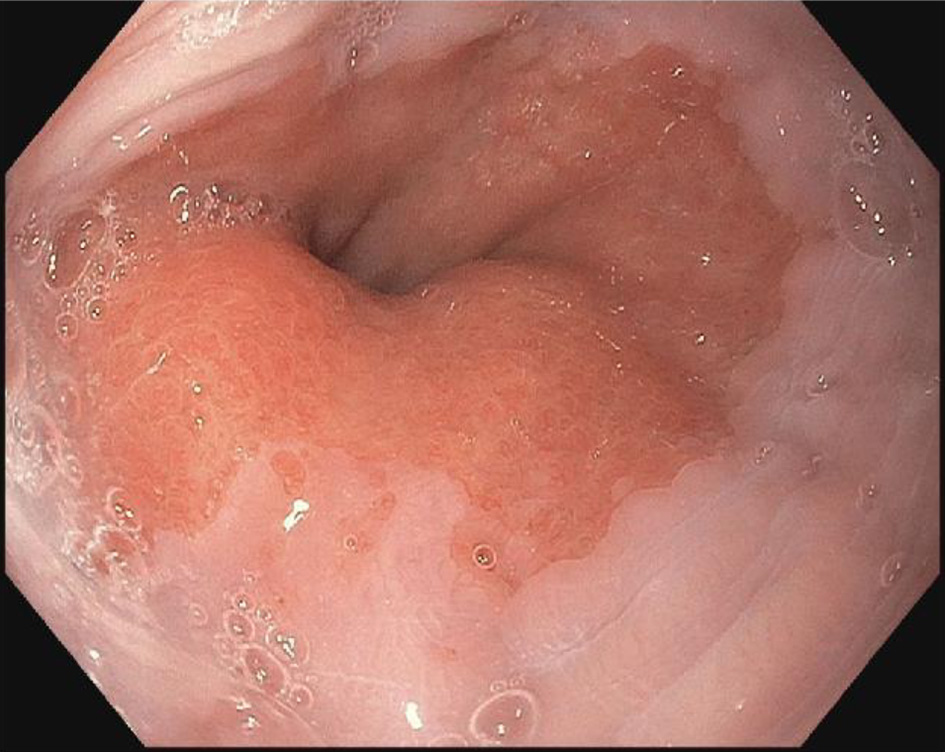 Click for large image | Figure 1. Endoscopy image of raised mucosa at gastroesophageal junction. |
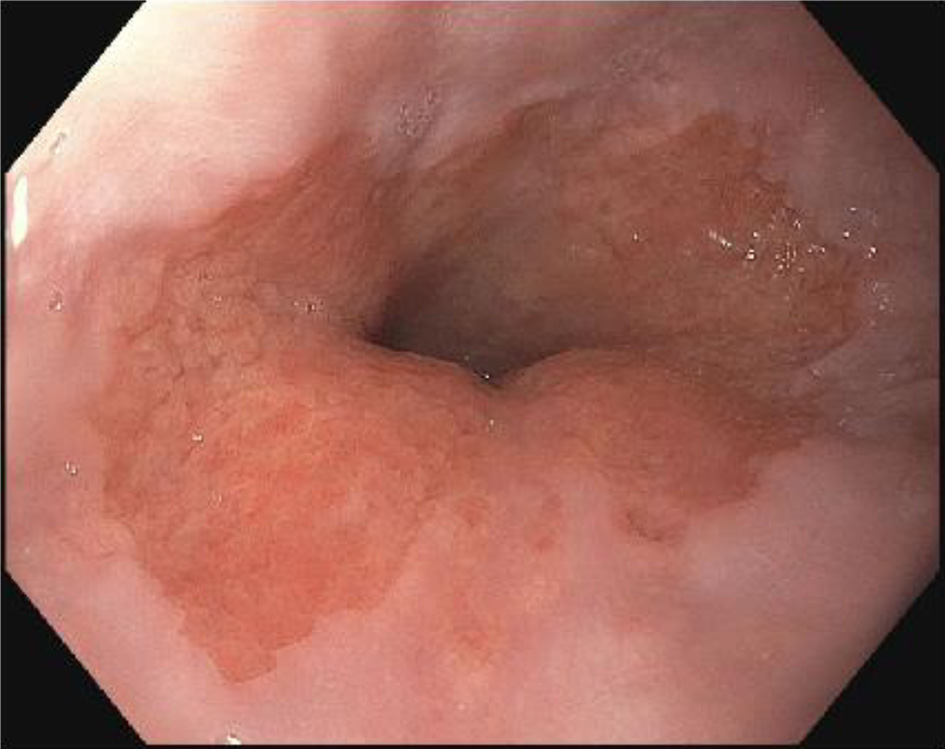 Click for large image | Figure 2. Irregular mucosa on endoscopic evaluation of gastroesophageal junction. |
The pathologist reported that histopathology of the GE junction biopsies revealed intestinal metaplasia and focal pancreatic acinar type tissue without dysplasia (Fig. 3). Additionally, stomach biopsies revealed H. pylori and intestinal metaplasia with active chronic gastritis (Fig. 4).
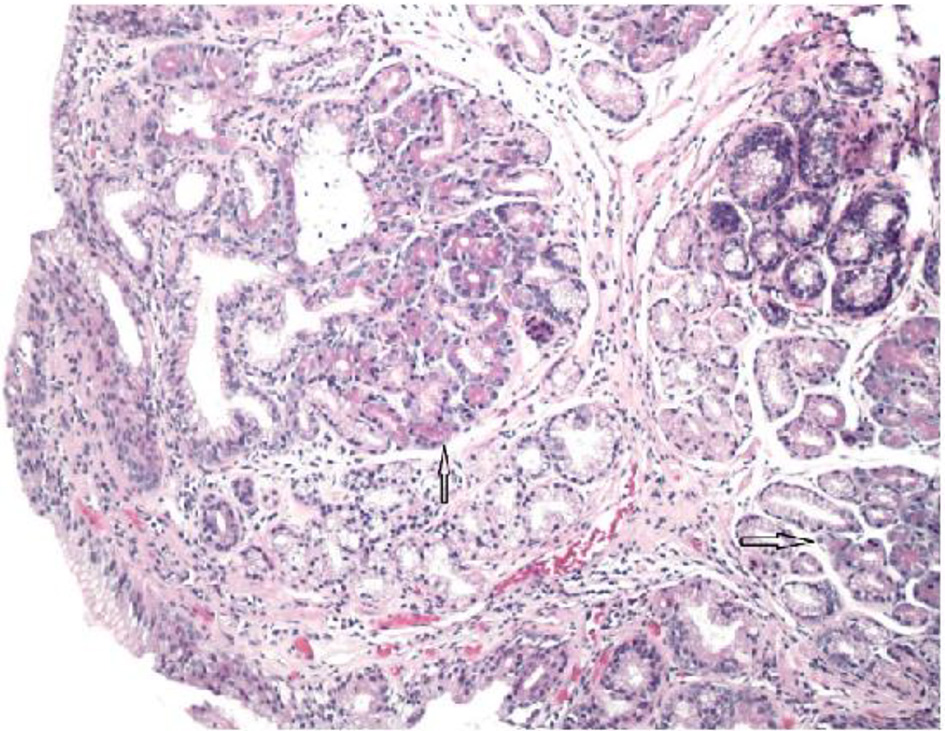 Click for large image | Figure 3. Glandular mucosa of esophagus with arrow pointing to focal pancreatic acinar tissue (H&E, × 100 magnification). |
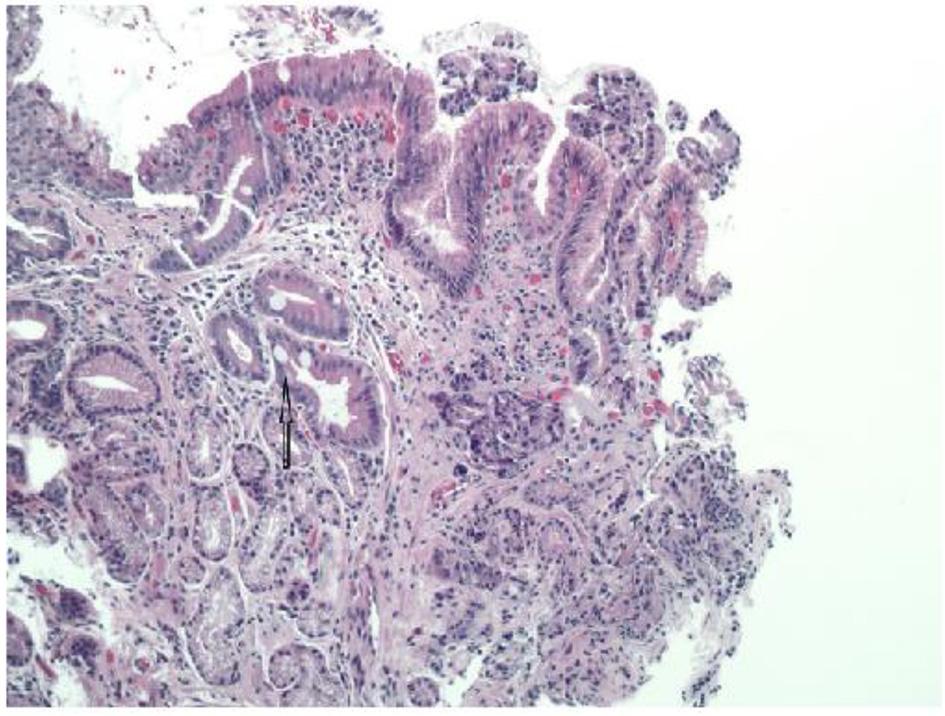 Click for large image | Figure 4. Glandular mucosa with arrow pointing to intestinal metaplasia (H&E, × 100 magnification). |
Follow-up and outcomes
At the follow-up gastroenterology clinic visit after the upper gastrointestinal endoscopy, the patient reported complete resolution of his dysphagia. Review of systems was significant for improvement in constipation with the use of the medications as prescribed. For this visit, he was prescribed antibiotics for H. pylori infection and scheduled for continued surveillance.
| Discussion | ▴Top |
Dysphagia, an upper gastrointestinal symptom, refers to the sensation of impaired passage of food from the mouth to the stomach that is caused by structural or neuromuscular conditions of the esophagus [1-7]. It has been estimated to affect 8-22% of persons above 50 years of age, with a higher prevalence in nursing homes [2, 4]. The prevalence of dysphagia in adults at least weekly was previously found to be 3% for men and women [7]. Based on the anatomical site of the esophagus that is primarily affected, dysphagia can be grouped as oropharyngeal or esophageal. The patient in this index case presented with esophageal dysphagia which may result from benign or malignant pathologies.
Benign causes of esophageal dysphagia include gastroesophageal reflux disease, peptic strictures, esophageal rings, eosinophilic esophagitis, diffuse esophageal spasm, achalasia and hypomotility [1-5]. It is important to exclude a premalignant or malignant etiology during the initial patient evaluation by conducting a thorough history and physical examination. The clinical suspicion for malignancy should be heightened by the presence of alarming features such as unintentional weight loss, dysphagia more for solids than for liquids, upper gastrointestinal bleeding, odynophagia, vomiting and onset age older than 50 years [3-5]. The patient in this index case presented with dysphagia with concomitant alarming features of weight loss, dysphagia to solids and older age. This increased the urgency to rule out a non-benign etiology of his gastrointestinal symptoms.
Guidelines recommend an empiric trial of a proton pump inhibitor (PPI) for patients who have esophageal dysphagia and reflux, the absence of the aforementioned alarming features [4-6]. For patients who have alarming features on their initial presentation or who have persistent gastrointestinal symptoms after empiric PPI therapy, endoscopy is recommended [4-6]. An EGD plays a diagnostic and therapeutic role in the evaluation of dysphagia. EGD allows for direct visualization of the esophageal lumen and stomach. Mucosal biopsies can also be taken for histopathology. It has been shown that EGD has a diagnostic yield of 54% when used in the initial evaluation of patients older than 40 years of age who present with dysphagia with associated heartburn, odynophagia or weight loss [5, 6].
EGD in this index case revealed a raised area with abnormal mucosa at the GE junction. Histopathology was consistent with focal EPAH. EPAH refers to histologically confirmed pancreatic tissue, or pancreatic rests, located within the esophagus [8-12]. EPAH lack any anatomic, neural or vascular connections to the main body of the orthotopic pancreas [9, 10, 12]. EPAH has been rarely reported in the literature. Pancreatic acinar heterotopia has been more commonly found in other areas of the gastrointestinal tract, with over 90% of previously reported cases occurring in the gastric antrum, duodenum, jejunum and ileum [9, 12, 13]. Pancreatic acinar heterotopia, of any location in the gastrointestinal tract, has been incidentally discovered in 0.5-13.7% of autopsies [14, 15], and 0.2-0.5% of laparotomies [14].
Though the exact pathogenesis of EPAH is not well-known, there have been suggested mechanisms. This includes theories such as the “migration theory” which supports an embryonic origin of EPAH. The formation of the pancreas begins at about 5 weeks into organogenesis when the ventral and dorsal pancreatic buds, which arise from the duodenal aspect of the foregut, rotate and fuse together [14, 16]. It has been postulated that the detachment of branching pancreatic buds during rotation of the foregut gives rise to heterotopic pancreatic tissue [17]. This may account for the prepyloric region, followed by other derivatives of the primitive foregut, being the most common location for these pancreatic rests [18, 19]. The stomach accounts for 25-38% of cases, followed by the duodenum and jejunum which account for 17-21% and 15-21%, respectively [15].
Uncomplicated heterotopic pancreatic rests present in the gastrointestinal tract do not typically cause gastrointestinal symptoms or manifestations. However, this pathology has been incidentally found in up to 24% of patients undergoing elective EGD for evaluation of upper gastrointestinal symptoms [10]. On direct visualization with endoscopy, the involved area of esophageal mucosa may often appear normal [9]. However, if the area of heterotopic pancreatic rest is large, it may then appear as a non-specific mass within the wall of gastrointestinal tract [15]. The appearance has also been described as a submucosal lesion with an associated central umbilication [8, 9]. In regards to the radiological appearance, on computed tomography imaging, the heterotopic pancreas has been described as an oval intramural mass with an endoluminal growth pattern and micro-lobulated margins [19].
For the existing cases of EPAH reported in the literature, it is twice as common in men which is similar to the patient in this case (Table 1 [9, 15] and Table 2 [11-13, 20-27]). Further, it is usually discovered in the fifth or sixth decade of life which is also consistent with this case presentation of 57-year-old man [20]. In most EPAH cases, the pancreatic rests are found in the distal third of the esophagus [9], and in our index case, the endoscopy revealed irregularities at the GE junction. One study demonstrated that when patients were divided into those with or without pancreatic acinar cells discovered on endoscopy, the patients with pancreatic acinar cells were slightly younger than the patients without (median 50 vs. 53 years; P = 0.009) [11]. In patients younger than 18 years of age, it has been associated with congenital anatomic abnormalities of the esophagus such as esophageal atresia, esophageal duplication cysts, tracheoesophageal fistula or esophageal duplication [9, 21]. The patient in our case did not have any known congenital abnormalities of his esophageal anatomy on presentation and none were found on endoscopy.
 Click to view | Table 1. Brief Literature Review of EPAH Case Series |
 Click to view | Table 2. Brief Literature Review of EPAH Reports |
Though symptoms are not usually associated with EPAH, they may occur secondary to a mass effect in large EPAH [9]. Gastrointestinal symptoms may include dysphagia, the presenting symptom of this index case, as well as abdominal pain localized to epigastric or right upper quadrant region [15]. However, the presence of dysphagia to solids only and unintentional weight loss were unusual clinical features. These symptoms usually suggest more sinister disorders of the esophagus such as malignant pathologies. Other symptoms that have been described in the literature from large EPAH include obstruction, intussusception and hemorrhage [9]. Malignant transformation of EPAH is reported to be uncommon [9, 11]. The heterotopic pancreas is thought to be as susceptible as the orthotopic pancreas to premalignant changes such as pancreatic intraepithelial neoplasia [13, 15]. However, to date, there has been only one reported case each of adenocarcinoma and anaplastic carcinoma arising from EPAH [15, 22, 23].
Management of EPAH has varied in the previously reported cases. Treatment options described in the literature have ranged from a conservative approach with clinical observation to a radical approach with surgical resections such as Ivor-Lewis esophagectomy. A minimally invasive surgical approach with left video-assisted thoracic surgery (VATS) was described in a case report in which the preceding esophageal biopsy specimens were non-diagnostic [27]. Surgical modalities have been recommended when endoscopy or imaging does not provide a pre-operative diagnosis. In these cases, complete surgical resection allows for tissue diagnosis. For malignant EPAH radical excision has been recommended [8, 27]. Enucleation has also been described for benign EPAH. In our index case, the EGD with biopsy was diagnostic. There were no endoscopic or histologic findings of malignancy. Further, endoscopic resection was therapeutic given the resolution of patient reported upper gastrointestinal symptoms.
Learning points
This case presentation serves to highlight EPAH as an uncommon cause of dysphagia, which is a common upper gastrointestinal complaint. Resection of EPAH during EGD treated the mass effect and associated gastrointestinal symptoms that were experienced by this patient. Current knowledge about the management for EPAH is limited due to its rare occurrence. Surgical resection has been recommended but this index case supports the effectiveness of endoscopic removal of EPAH. The presence of intestinal metaplasia on histology raises the question of whether this finding is to be considered a benign or premalignant finding in patients. Continued surveillance with the patient in our case will allow for monitoring of symptoms, including any concerning for a malignant transformation. Continued research on the endoscopic findings and histological findings of EPAH may better characterize this condition and the likelihood of risks such as malignant transformation.
Acknowledgments
This case was submitted for the American College of Gastroenterology Annual Scientific Meeting 2019; Abstract published in The American Journal of Gastroenterology 114(Supplement):S1664.
Financial Disclosure
The authors declare no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors declare no potential conflict of interest.
Informed Consent
Written informed consent was obtained from the patient for his anonymized information to be published in this article.
Author Contributions
KM, TS and TG: manuscript planning and preparation, literature review, and editing of subsequent revisions. MS: diagnosis and management of the patient, designed research and provided expert clinical knowledge.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
EPAH: esophageal pancreatic acinar heterotopia; GEJ: gastroesophageal junction; DE: distal esophagus; ME: middle esophagus; PE: proximal esophagus; PPI: proton pump inhibitor; EGD: esophagogastroduodenoscopy
| References | ▴Top |
- Dysphagia. American College of Gastroenterology. https://gi.org/topics/dysphagia/. Accessed April 16, 2022.
- Lind CD. Dysphagia: evaluation and treatment. Gastroenterol Clin North Am. 2003;32(2):553-575.
doi - Malagelada JR, Bazzoli F, Boeckxstaens G, De Looze D, Fried M, Kahrilas P, Lindberg G, et al. World gastroenterology organisation global guidelines: dysphagia—global guidelines and cascades update September 2014. J Clin Gastroenterol. 2015;49(5):370-378.
doi pubmed - Liu LWC, Andrews CN, Armstrong D, Diamant N, Jaffer N, Lazarescu A, Li M, et al. Clinical practice guidelines for the assessment of uninvestigated esophageal dysphagia. J Can Assoc Gastroenterol. 2018;1(1):5-19.
doi pubmed - Asge Standards of Practice Committee, Pasha SF, Acosta RD, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, et al. The role of endoscopy in the evaluation and management of dysphagia. Gastrointest Endosc. 2014;79(2):191-201.
doi pubmed - Varadarajulu S, Eloubeidi MA, Patel RS, Mulcahy HE, Barkun A, Jowell P, Libby E, et al. The yield and the predictors of esophageal pathology when upper endoscopy is used for the initial evaluation of dysphagia. Gastrointest Endosc. 2005;61(7):804-808.
doi - Cho SY, Choung RS, Saito YA, Schleck CD, Zinsmeister AR, Locke GR, 3rd, Talley NJ. Prevalence and risk factors for dysphagia: a USA community study. Neurogastroenterol Motil. 2015;27(2):212-219.
doi pubmed - Qualia CM, Rossi TM, Ullah A. Heterotopic pancreatic tissue found in the esophagus of a 14-year-old girl. Gastroenterol Hepatol (N Y). 2007;3(12):939-940.
- Wang HH, Zeroogian JM, Spechler SJ, Goyal RK, Antonioli DA. Prevalence and significance of pancreatic acinar metaplasia at the gastroesophageal junction. Am J Surg Pathol. 1996;20(12):1507-1510.
doi pubmed - Popiolek D, Kahn E, Markowitz J, Daum F. Prevalence and pathogenesis of pancreatic acinar tissue at the gastroesophageal junction in children and young adults. Arch Pathol Lab Med. 2000;124(8):1165-1167.
doi pubmed - Crighton E, Botha A. Intraductal papillary mucinous neoplasm of the oesophagus: an unusual case of dysphagia. Ann R Coll Surg Engl. 2012;94(2):e92-94.
doi pubmed - Mack T, Lowry D, Carbone P, Barbick B, Kindelan J, Marks R. Multimodality imaging evaluation of an uncommon entity: esophageal heterotopic pancreas. Case Rep Radiol. 2014;2014:614347.
doi pubmed - Ulrych J, Fryba V, Skalova H, Krska Z, Krechler T, Zogala D. Premalignant and malignant lesions of the heterotopic pancreas in the esophagus: a case report and review of the literature. J Gastrointestin Liver Dis. 2015;24(2):235-239.
doi pubmed - Wlaz J, Madro A, Kazmierak W, Celinski K, Slomka M. Pancreatic and gastric heterotopy in the gastrointestinal tract. Postepy Hig Med Dosw (Online). 2014;68:1069-1075.
doi pubmed - Schneider NI, Plieschnegger W, Geppert M, Wigginghaus B, Hoss GM, Eherer A, Wolf EM, et al. Pancreatic acinar cells—a normal finding at the gastroesophageal junction? Data from a prospective Central European multicenter study. Virchows Arch. 2013;463(5):643-650.
doi pubmed - Robbins SL, Kumar V. Robbins and cotran pathologic basis of disease. 8th ed. Philadelphia, PA: Saunders/Elsevier; 2010.
- Trifan A, Tarcoveanu E, Danciu M, Hutanasu C, Cojocariu C, Stanciu C. Gastric heterotopic pancreas: an unusual case and review of the literature. J Gastrointestin Liver Dis. 2012;21(2):209-212.
- Kim JY, Lee JM, Kim KW, Park HS, Choi JY, Kim SH, Kim MA, et al. Ectopic pancreas: CT findings with emphasis on differentiation from small gastrointestinal stromal tumor and leiomyoma. Radiology. 2009;252(1):92-100.
doi pubmed - Rezvani M, Menias C, Sandrasegaran K, Olpin JD, Elsayes KM, Shaaban AM. Heterotopic pancreas: histopathologic features, imaging findings, and complications. Radiographics. 2017;37(2):484-499.
doi pubmed - Noffsinger AE, Hyams DM, Fenoglio-Preiser CM. Esophageal heterotopic pancreas presenting as an inflammatory mass. Dig Dis Sci. 1995;40(11):2373-2379.
doi pubmed - Salo JA, Dlouhy M, Virtanen I. Congenital cyst and heterotopic pancreatic tissue in the oesophagus. Ann Chir Gynaecol. 1993;82(4):263-265.
- Guillou L, Nordback P, Gerber C, Schneider RP. Ductal adenocarcinoma arising in a heterotopic pancreas situated in a hiatal hernia. Arch Pathol Lab Med. 1994;118(5):568-571.
- Roshe J, Del Buono E, Domenico D, Colturi TJ. Anaplastic carcinoma arising in ectopic pancreas located in the distal esophagus. J Clin Gastroenterol. 1996;22(3):242-244.
doi pubmed - Razi MD. Ectopic pancreatic tissue of esophagus with massive upper gastrointestinal bleeding. Arch Surg. 1966;92(1):101-104.
doi pubmed - Yamagiwa I, Obata K, Ouchi T, Sotoda Y, Shimazaki Y. Heterotopic pancreas of the esophagus associated with a rare type of esophageal atresia. Ann Thorac Surg. 1998;65(4):1143-1144.
doi - Gananadha S, Hunt DR. A unique case of pancreatitis and retention cyst in esophageal heterotopic pancreas. Surg Laparosc Endosc Percutan Tech. 2005;15(6):345-347.
doi pubmed - Lowry DM, Mack TE, Partridge BJ, Barbick BC, Marks RM, Kindelan JT. Thorascopic resection of esophageal heterotopic pancreas. Ann Thorac Surg. 2013;96(5):1850-1851.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


