| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 8, August 2022, pages 380-385
Functionality of Monoclonal Antibody Therapy in SARS-CoV-2
Ruhma Alia, c, Aditya Patela, c, Muhammad A. Waqasa, Krunal Trivedia, Jihad Slimb
aDepartment of Internal Medicine, Saint Michael’s Medical Center, Newark, NJ, USA
bDepartment of Infectious Disease, Saint Michael’s Medical Center, Newark, NJ, USA
cCorresponding Author: Ruhma Ali and Aditya Patel, Department of Internal Medicine, Saint Michael’s Medical Center, Newark, NJ 07102, USAand
Manuscript submitted June 9, 2022, accepted July 11, 2022, published online August 19, 2022
Short title: Functionality of mAb Therapy in SARS-CoV-2
doi: https://doi.org/10.14740/jmc3968
| Abstract | ▴Top |
The coronavirus disease 2019 (COVID-19) pandemic emerged as a world crisis in 2019 and started a global search for optimal therapeutic regimen including vaccines, antiviral agents, and recently monoclonal antibody therapy. Clinical trials are currently underway for the efficacy of several neutralizing monoclonal antibodies against COVID-19. The evolution of new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants with immune evasion capacity has created a challenge for the healthcare workers with urgent need for prospective studies to determine functionality of monoclonal antibody therapy and their role in the reduction of hospitalization for disease severity. Herein, we report three cases of COVID-19 during the beginning of the spread of Omicron variants that were hospitalized after treatment with monoclonal antibody therapy in the emergency department. All the patients showed progression of the disease on imaging and were treated with dexamethasone, remdesivir and anticoagulation based on the symptoms and contraindications. Two of the patients recovered and were discharged with out-patient follow-up; however, one patient expired in the hospital. Monoclonal antibody therapy is a promising treatment to limit the progression of COVID-19 and reduce the hospital strain specifically in small community hospitals. Limited information is available about their efficacy in the new viral variants. These cases emphasize the need of future prospective study and randomized controlled trials to illustrate the utilization of monoclonal antibodies as a therapeutic modality in patients infected with the variants of SARS-CoV-2.
Keywords: Monoclonal antibody therapy; COVID-19 treatment; Omicron; Viral variants; Chest X-ray
| Introduction | ▴Top |
The current pandemic caused by novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has claimed the lives of millions of people worldwide [1]. To combat this contagious disease, a variety of prophylactic and therapeutic treatment regimens are now under evaluation in clinical trials [2]. Immunotherapy in the form of vaccines have been proven to be beneficial in viral infections. Monoclonal antibodies (mAbs) represent a form of passive immunotherapy that can provide effective prophylactic and therapeutic intervention against specific diseases [3]. These are a novel class of antiviral agents that can neutralize the virus in infected patients [4]. In 1975, mAbs were first developed by Kohler and Milstein with palivizumab being the first antiviral mAb approved for the prophylaxis of respiratory syncytial virus in high-risk infants by the United States Food and Drug Administration (FDA) [5]. The early stage of coronavirus disease 2019 (COVID-19) is characterized by profound SARS-CoV-2 viral replication and the use of antibody-based treatments are likely to be beneficial in this stage. Here in, we report three cases in which patients with suspected Omicron variant of SARS-CoV-2 were treated with mAbs and hospitalized for progression of disease.
| Case Reports | ▴Top |
Case 1
Investigations
A 75-year-old female with a past medical history (PMH) of diabetes mellitus, hypertension and peripheral arterial disease presented to the emergency department (ED) on December 13, 2021 with complaints of loss of appetite and cough for the past 3 days. The patient stated the cough was dry, non-productive and non-bloody. The patient denied any weight loss, headache, vomiting, diarrhea, fever, chills, or shortness of breath. She is not vaccinated for COVID-19. Medications include carvedilol 12.5 mg twice daily, aspirin 81 mg once daily, atorvastatin 40 mg once at night and insulin. The patient is compliant with her medications with no change in use or dosage of current medications. She did not report any change or exacerbation of her chronic medical conditions. On examination the patient was afebrile with temperature of 37 °C, pulse 78 beats/min (bpm), respiratory rate (RR) 18 breaths/min, blood pressure (BP) 169/64 mm Hg, saturating 100% on room air (RA). Cardiac, respiratory, and abdominal examination were within normal limits.
Diagnosis
On laboratory evaluation, COVID-19 antigen and polymerase chain reaction (PCR) tests were positive. The high sensitivity troponin (HS troponin) and creatinine were elevated. The remainder of the laboratory parameters were unremarkable. Electrocardiography showed sinus rhythm. The chest X-ray showed no infiltrates as shown in Figure 1. The patient received bamlanivimab 700 mg and etesevimab 1,400 mg transfusion and was discharged. The patient returned to the ED after 6 days for persistent loss of appetite and cough. She stated that the cough had worsened, was dry and non-productive. She denied any fever, chills, shortness of breath, chest pain, palpitations, abdominal pain, nausea, or diarrhea. Review of systems was otherwise negative. She was afebrile with a temperature of 36.8 °C, pulse of 72 bmp, RR 17 breaths/min, BP of 156/69 mm Hg, saturating 96% on RA. Physical examination including the respiratory and cardiac examination was unremarkable. The COVID-19 antigen test was negative, but the PCR test was positive. The inflammatory markers including ferritin, D-dimer, lactate dehydrogenase (LDH) were elevated. Chest X-ray showed multifocal airspace disease as shown in Figure 2. Computed tomography (CT) scan of chest without contrast showed bilateral peripheral multifocal consolidation. Doppler venous ultrasound of bilateral lower extremity was negative for acute deep vein thrombosis.
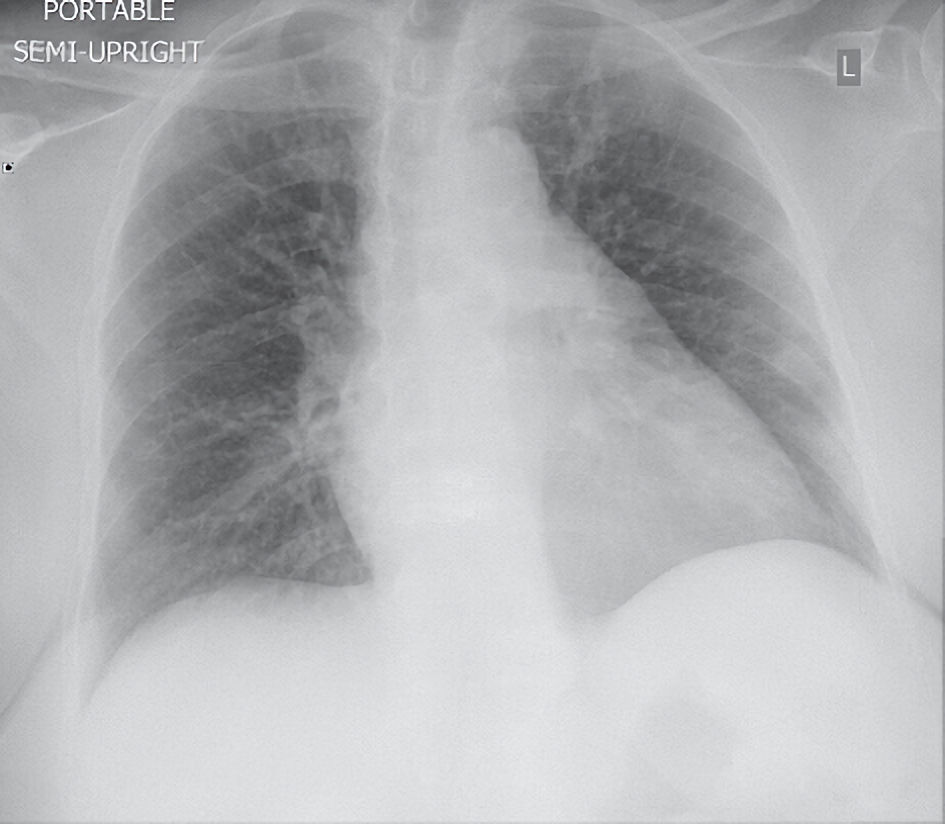 Click for large image | Figure 1. Posteroanterior chest X-ray showed no infiltrate, effusion, or pneumothorax. |
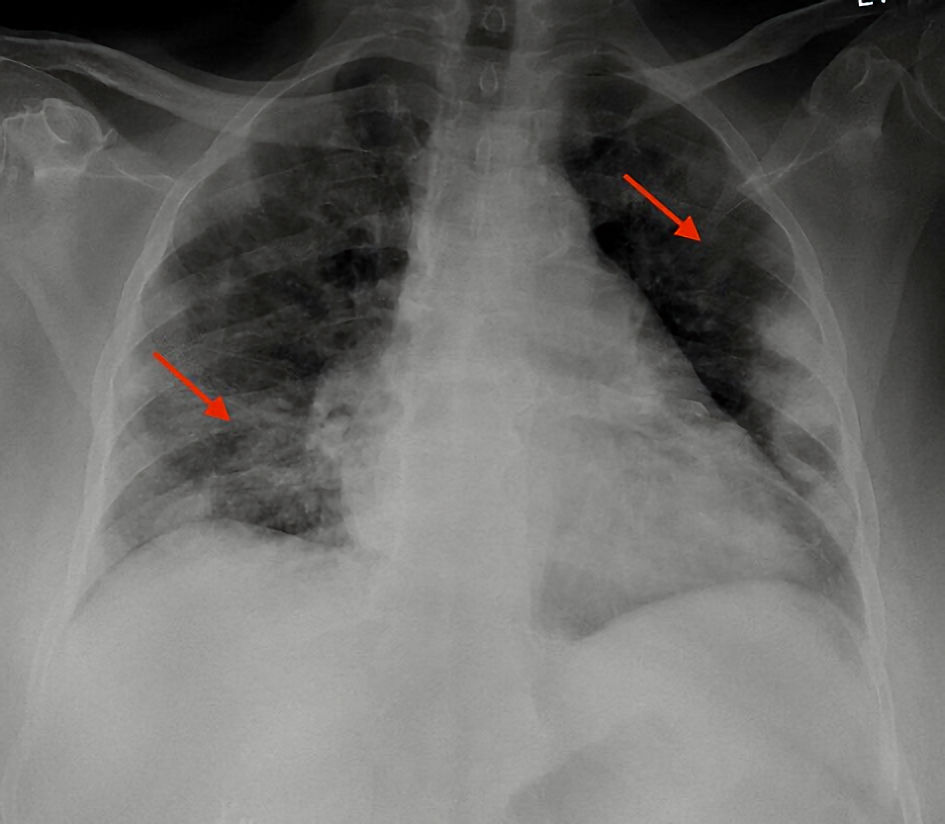 Click for large image | Figure 2. Posteroanterior chest X-ray showed multifocal airspace opacity bilaterally (red arrows). |
Treatment
The patient was given a 3-day course of remdesivir and prophylactic anticoagulation. Her cough and appetite improved during the hospital stay and she was discharged to a skilled nursing facility on a month of 5 mg apixaban daily with out-patient follow-up.
Follow-up and outcomes
On the follow-up visit after 2 weeks the patient reported recovery of her symptoms.
Case 2
Investigations
A 75-year-old male with a PMH of cirrhosis secondary to hepatitis C, acquired immunodeficiency syndrome (AIDS) and end stage renal disease (ESRD) on dialysis presented to the ED on December 21, 2021 from a long-term care facility with complaints of cough for the past 2 days. The patient stated the cough was non-bloody and productive of yellowish sputum. He denied any shortness of breath, fever, or chills. His past medical conditions were reported to be stable with regular follow-up in the facility. Medications include abacavir-lamivudine, fosamprenavir with CD4 count of 354 and unknown viral load with no change in the dosage or use of current medication. The review of the system was otherwise unremarkable. He received two doses of COVID-19 vaccination 8 months ago but did not receive the booster dose. Patient was unaware of which type of vaccine. On examination the patient was afebrile with a temperature of 36.6 °C, pulse 70 bpm, RR 20 breaths/min, saturating 95% on RA. The patient appeared alert on physical examination. The chest was clear to auscultation bilaterally with labored speech and respiration. The rest of the physical examination was within normal limits.
Diagnosis
The laboratory parameters were within normal limits. The PCR test for COVID-19 was positive, but the antigen test was negative. The chest X-ray was positive for hilar vascular congestion as shown in Figure 3. The patient was given casirivimab-imdevimab 1,200 mg mAb treatment in the ED and was discharged back to the facility. The patient presented back to the ED for worsening shortness of breath after 18 days. As per the facility nurse the patient had increased cough and shortness of breath in the past week. He was placed on a high flow nasal cannula in the facility. On evaluation in the ED the patient was in moderate respiratory distress and was unable to answer questions comprehensively. Patient was afebrile with a temperature of 36.8 °C with a pulse of 73 bmp and RR of 28 breaths/min, BP of 122/61 mm Hg, saturating 95% on continuous bilevel positive airway pressure (BiPAP) device. On examination the patient had bilateral crackles with poor air entry bilaterally. The remainder of the physical examination was unremarkable. The inflammatory markers including ferritin, D-dimer and LDH were markedly elevated. The COVID-19 antigen and PCR tests were negative. The brain natriuretic peptide (BNP) was elevated. The electrocardiogram showed sinus rhythm. The chest X-ray showed extensive bilateral infiltrates as shown in Figure 4. Echocardiography showed left ventricular ejection fraction (LVEF) of 50-55%. His initial arterial blood gas (ABG) showed a pH of 7.446 (7.34 - 7.44), pCO2 of 44.4 (35 - 48 mm Hg), pO2 of 45.7 (75 - 100 mm Hg) and bicarb of 29.8 (22 - 28 mmol/L). Doppler venous ultrasound of bilateral lower extremity was negative for acute deep vein thrombosis. The blood cultures were negative.
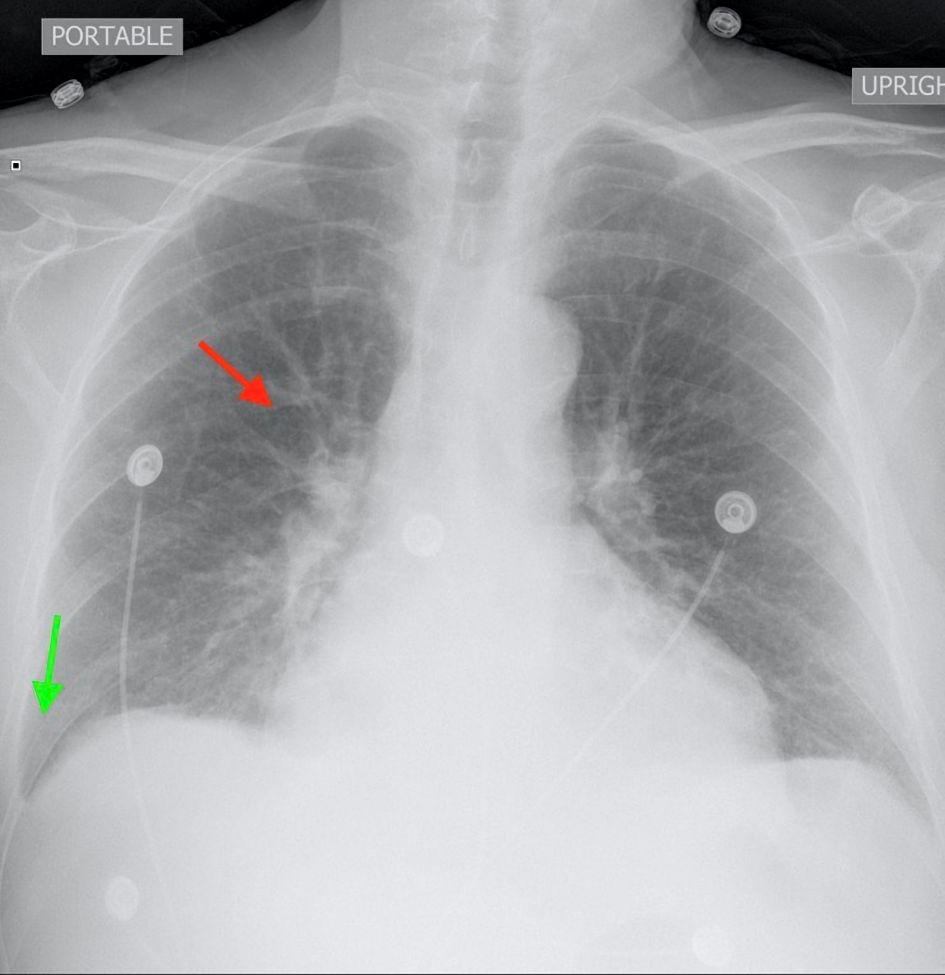 Click for large image | Figure 3. Posteroanterior chest X-ray showed hilar vascular congestion (red arrow) with mild right sided pleural effusion (green arrow). |
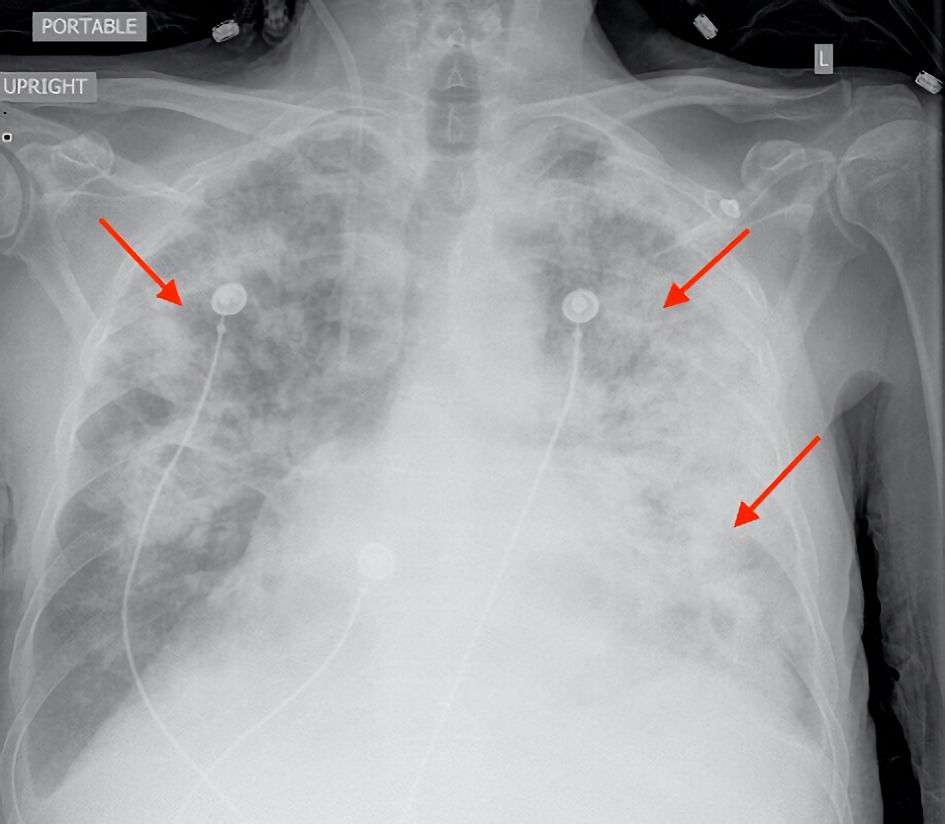 Click for large image | Figure 4. Posteroanterior chest X-ray showed extensive bilateral infiltrates (red arrows) and small effusion. |
Treatment
The patient was admitted to the intensive care unit (ICU) for increasing oxygen requirements. He was started on dexamethasone and prophylactic anticoagulation.
Follow-up and outcomes
During ICU stay the patient’s BP decreased, and he was started on pressor support. Goals of care were discussed with the patient who did not wish to be intubated. The code status was confirmed as do not resuscitate, do not intubate. His oxygen requirements continued to increase, and he expired on day 10.
Case 3
Investigations
A 54-year-old female with no significant PMH presented to the ED on December 27, 2021 with complaints of fatigue and loss of appetite for the past 4 days. She complained of mild shortness of breath with dry, non-bloody cough and 2 - 3 episodes of loose stools. The patient is not vaccinated for COVID-19. She denied any headache, chest pain, palpitations, or abdominal pain. The patient was afebrile with pulse 82 bmp, RR 20 breaths/min, saturating 97% on RA and body mass index (BMI) of 31 kg/m2. The physical examination was unremarkable.
Diagnosis
The COVID-19 antigen and PCR tests were positive. The remainder of the laboratory investigations were within normal limits. The chest X-ray showed mild pulmonary vascular congestion as shown in Figure 5. The patient was given casirivimab-imdevimab 1200 mg mAb treatment in the ED and was discharged, with instructions to self-isolate. The patient returned to the ED 4 days later with complaints of shortness of breath. She stated she felt short of breath on exertion. She also complained of a cough producing yellowish sputum. She still complained of loss of appetite and stated that she had fever and chills at home. She denied any chest pain, palpitations, headache, dizziness, nausea, or abdominal pain. Review of systems was otherwise negative. On evaluation she was febrile with Tmax (the maximum recorded temperature at a given station on a given day) of 39 °C , BP of 129/66 mm Hg, RR 18 breaths/min, pulse 97 bpm, saturating 92% on RA. On respiratory examination the patient had diffuse rales bilaterally. The remainder of the physical examination was within normal limits. The inflammatory markers were mildly elevated. The patient had leukopenia and thrombocytopenia. The other laboratory parameters were unremarkable as shown in Table 1. The chest X-ray showed diffuse patchy airspace disease as shown in Figure 6.
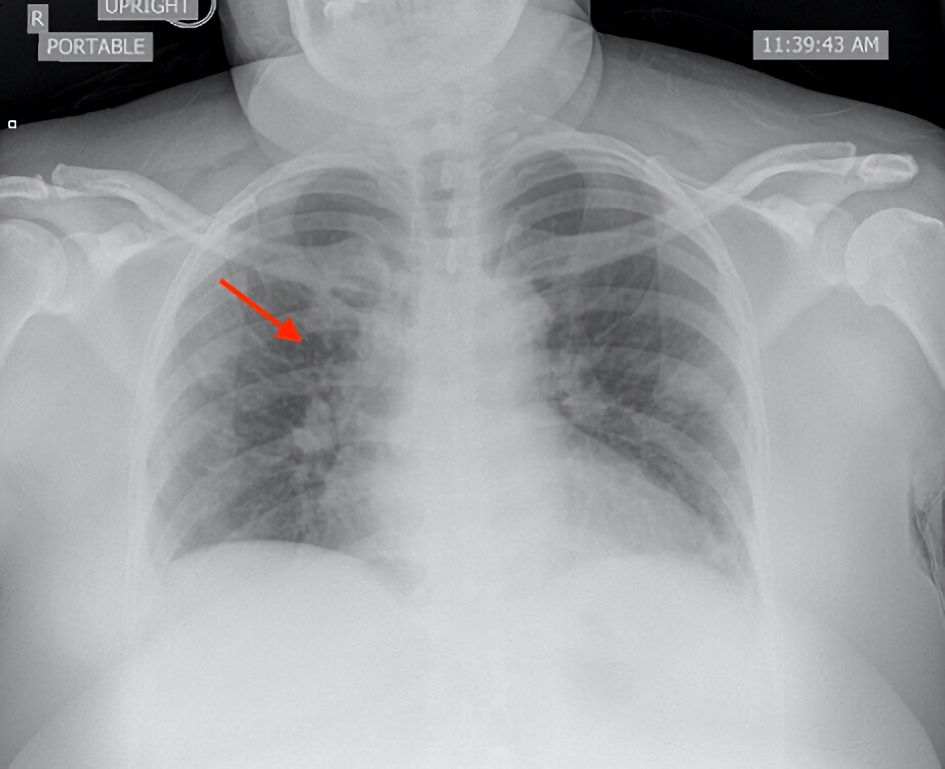 Click for large image | Figure 5. Posteroanterior chest X-ray showed mild vascular congestion (red arrow). No consolidation or infiltrate was observed. |
 Click to view | Table 1. Basic Laboratory Parameters |
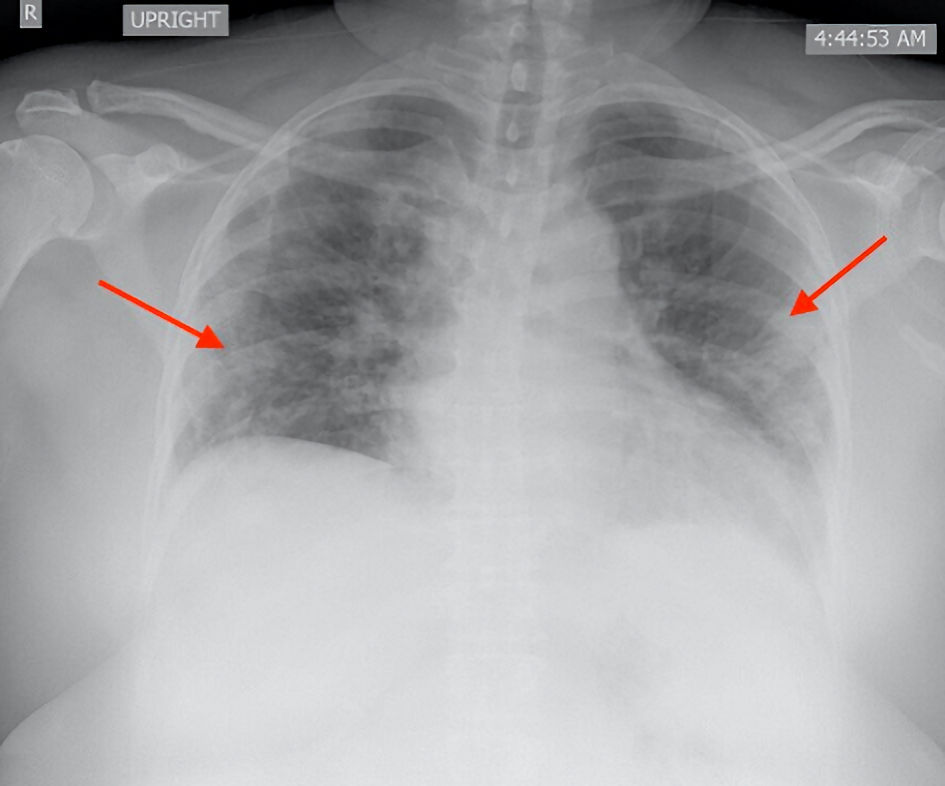 Click for large image | Figure 6. Posteroanterior chest X-ray showed mild diffuse patchy airspace opacity with peripheral distribution (red arrow). |
Treatment
She was given a 7-day course of dexamethasone, 5 days of remdesivir and prophylactic anticoagulation. The patient’s oxygen requirements improved, and she was discharged on apixaban 2.5 mg daily for 2 weeks with out-patient follow-up.
Follow-up and outcomes
On the subsequent visit the patient denied any active complaints.
| Discussion | ▴Top |
The COVID-19 pandemic has caused an unprecedented public health crisis globally and warrants the timely creation of optimal preventive and therapeutic interventions [6]. SARS-CoV-2 primarily affects the organs with a high quantity of virus targeted receptor protein: angiotensin converting enzyme 2 (ACE2) which includes the lungs, heart, kidney, and small intestine [7]. Clinical manifestations include mild symptoms like diarrhea, shortness of breath and cough to severe complications including acute respiratory distress syndrome, widespread thrombosis, renal failure, and death [8]. Current treatment modalities for moderate and severe for COVID-19 include antiviral agents, immunosuppressants, corticosteroids, anticoagulants, and supportive management [9].
Recently mAbs have emerged as a beneficial tool for the prophylactic and therapeutic treatment of COVID-19 based on their specificity, ability to prevent disease progression and reduction of disease period [10]. It follows the hypothesis that passive immunotherapy might be useful for the management of COVID-19 since patients who develop antibodies soon after disease occurrence have better prognosis than those with delayed antibody response [11]. The mAbs are derived from the B cells. These antibodies neutralize the virus by binding to the spike proteins of the SARS-CoV-2 and block the entry of the virus into the host cells thereby reducing the viral load [12]. The FDA has issued an emergency use authorization (EUA) of mAbs for the treatment of mild to moderate COVID-19 disease within 10 days of symptom onset including bamlanivimab-etesevimab, casirivimab-imdevimab, bebtelovimab and sotrovimab; however, the use of bamlanivimab-etesevimab and casirivimab-imdevimab against Omicron variant was later revised in January 2022 due to lack of efficacy against the variant. The combination of casirivimab and imdevimab (REGN-COV2) binds to the epitopes of the spike protein for SARS-CoV-2 and blocks the virus binding to the human receptors causing decrease in the viral load [3]. Bamlanivimab-etesevimab also has a similar mechanism of action and can be used in patients with mild to moderate symptoms and high risk of progression to severe disease [13]. Clinical trials for the efficacy, rate of hospitalization and mortality benefits of mAbs for COVID-19 are currently underway for patients with early disease [14]. However, limited benefit has been observed in patients with severe disease [13].
A study conducted by Bariola et al showed that 15 out of 232 propensity matched patients were hospitalized after treatment with bamlanivimab and only four of these patients died [15]. This study emphasized that bamlanivimab therapy reduced the hospitalization and mortality in patients more than 65 years of age with COVID-19. Another study conducted by Gupta et al showed that only three out of 291 patients with COVID-19 treated with sotrovimab had disease progression leading to hospitalization and death [16]. The emergence of viral variants, however, is an issue of concern since it may undermine the clinical effectiveness of current mAbs therapy [17]. Omicron (B1.1.529) is a new variant with missense substitutions in the spike protein receptor domain that might not be neutralized by casirivimab and imdevimab as per unpublished in vitro studies [18]. If this hypothesis proves true, then it will significantly compromise the efficacy of mAbs against the Omicron variant [19]. The National Institutes of Health (NIH) COVID-19 treatment guidelines also recommend against the use of bamlanivimab-etesevimab and casirivimab-imdevimab for Omicron variant and as such have revoked the EUA of these mAbs due to lack of efficacy against the Omicron variant as of January 2022 [20].
We compiled the data of 125 patients with mild and moderate symptoms of COVID-19 who received mAbs over the course of 2 months from October 2021 to December 2021. Twelve patients out of 125 received sotrovimab whereas 55 received casirivimab/imdevimab and 58 received bamlanivimab/etesevimab. Most of the patients were Hispanic. Diabetes and hypertension were the most common comorbidities. Majority of the patients were male. We report three cases of hospitalization out of these 125 patients post mAbs treatment. One patient received bamlanivimab 700 mg and etesevimab 1,400 mg transfusion whereas the remaining two received casirivimab-imdevimab 1,200 mg mAb treatment. Out of the three, one patient who received casirivimab-imdevimab, passed away due to progression of disease. It is to be noted that the patient had multiple comorbidities which might be a cofounder to the efficacy of mAbs. All patients were above 54 years of age. Two patients were hospitalized within a week of mAb administration whereas one patient was hospitalized after 2 weeks for worsening clinical symptoms. Only one patient was upgraded to the ICU due to progression of the disease. All the patients were given prophylactic anticoagulation whereas, only two patients received remdesivir and dexamethasone. One patient had no significant PMH except obesity whereas the other two had multiple comorbidities. All of the patients showed progression of disease on imaging; however, the only patient with unfavorable outcome had significant co-morbidities which might have played a role in the severity of his symptoms. A study conducted by Lovett et al demonstrated that out of 270 patients treated with bamlanivimab only five patients (1.9%) required hospitalization and the risk of hospitalization was 82% lower in mAb treated patients as compared to untreated patients [20]. These cases coincide with the beginning of the spread of the Omicron variant and even though the viral genome was not sequenced due to the paucity of funds in the community hospital, the timing of the cases suggest infection with the Omicron variant and the therapeutic failure of the mAbs might be due to the reduced activity of mAbs against the newer variant. It is to be noted that mAbs are susceptible to mutations and not useful for permanent intervention.
Future prospective studies are required to explore medical conditions and viral variants that may benefit from mAbs for the treatment of COVID-19. Outpatient strategies to decrease the rate of hospitalization and death are imperative to reduce the disease burden caused by SARS-CoV-2 and as such also supports the investment of funds for the development of infusion infrastructure.
Learning points
The use of neutralizing antibodies has a potential utility as a treatment option for COVID-19 and clinical trials are underway to determine the optimum strategic use. The emergence of viral variants is a limiting factor for the treatment with mAbs and should be kept in consideration while using the mAbs.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Written informed consents were obtained from the patients for publication of the case report.
Author Contributions
RA, AP, MW, and KT contributed to diagnosis and management of the patient, discussion, writing and drafting of the case, final approval of the case reports. JS contributed to management of the patient, revision, and final approval of the case reports.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- World Health Organization. WHO COVID-19 dashboard. World Health Organization. Published 2022. https://covid19.who.int/.
- Taylor PC, Adams AC, Hufford MM, de la Torre I, Winthrop K, Gottlieb RL. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat Rev Immunol. 2021;21(6):382-393.
doi pubmed - Deb P, Molla MMA, Saif-Ur-Rahman KM. An update to monoclonal antibody as therapeutic option against COVID-19. Biosaf Health. 2021;3(2):87-91.
doi pubmed - Renn A, Fu Y, Hu X, Hall MD, Simeonov A. Fruitful neutralizing antibody pipeline brings hope to defeat SARS-Cov-2. Trends Pharmacol Sci. 2020;41(11):815-829.
doi pubmed - Aleem A, Slenker AK. Monoclonal antibody therapy for high-risk coronavirus (COVID 19) patients with mild to moderate disease presentations. In: StatPearls. Treasure Island (FL), 2022.
- Patel A, Ajayi F, Ali R, Chan KH, Slim J. Dilemma of Anticoagulation Therapy in Mild or Asymptomatic COVID-19 Cases. Cureus. 2021;13(11):e19291.
doi - Hwang YC, Lu RM, Su SC, Chiang PY, Ko SH, Ke FY, Liang KH, et al. Monoclonal antibodies for COVID-19 therapy and SARS-CoV-2 detection. J Biomed Sci. 2022;29(1):1.
doi pubmed - Ali R, Patel A, Chan KH, Veeraballi S, Slim J. A case series of SARS-CoV-2 and influenza co-infection. Cureus. 2021;13(8):e17597.
doi - Molhave M, Agergaard J, Wejse C. Clinical management of COVID-19 patients - an update. Semin Nucl Med. 2022;52(1):4-10.
doi pubmed - Lu RM, Chiu CY, Liu IJ, Chang YL, Liu YJ, Wu HC. Novel human Ab against vascular endothelial growth factor receptor 2 shows therapeutic potential for leukemia and prostate cancer. Cancer Sci. 2019;110(12):3773-3787.
doi pubmed - Lucas C, Klein J, Sundaram ME, Liu F, Wong P, Silva J, Mao T, et al. Author Correction: Delayed production of neutralizing antibodies correlates with fatal COVID-19. Nat Med. 2021;27(7):1309.
doi pubmed - Jones BE, Brown-Augsburger PL, Corbett KS, Westendorf K, Davies J, Cujec TP, Wiethoff CM, et al. The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates. Sci Transl Med. 2021;13(593):eabf1906.
doi pubmed - Coronavirus (COVID-19) Update: FDA authorizes monoclonal antibody for treatment of COVID-19. FDA. Published November 9, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19.
- Webb BJ, Buckel W, Vento T, Butler AM, Grisel N, Brown SM, Peltan ID, et al. Real-world effectiveness and tolerability of monoclonal antibody therapy for ambulatory patients with early COVID-19. Open Forum Infect Dis. 2021;8(7):ofab331.
doi pubmed - Bariola JR, McCreary EK, Wadas RJ, Kip KE, Marroquin OC, Minnier T, Koscumb S, et al. Impact of bamlanivimab monoclonal antibody treatment on hospitalization and mortality among nonhospitalized adults with severe acute respiratory syndrome coronavirus 2 infection. Open Forum Infect Dis. 2021;8(7):ofab254.
doi pubmed - Gupta A, Gonzalez-Rojas Y, Juarez E, Crespo Casal M, Moya J, Falci DR, Sarkis E, et al. Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385(21):1941-1950.
doi pubmed - Taha Y, Wardle H, Evans AB, Hunter ER, Marr H, Osborne W, Bashton M, et al. Persistent SARS-CoV-2 infection in patients with secondary antibody deficiency: successful clearance following combination casirivimab and imdevimab (REGN-COV2) monoclonal antibody therapy. Ann Clin Microbiol Antimicrob. 2021;20(1):85.
doi pubmed - Petersen E, Ntoumi F, Hui DS, Abubakar A, Kramer LD, Obiero C, Tambyah PA, et al. Emergence of new SARS-CoV-2 variant of concern omicron (B.1.1.529) - highlights Africa's research capabilities, but exposes major knowledge gaps, inequities of vaccine distribution, inadequacies in global COVID-19 response and control efforts. Int J Infect Dis. 2022;114:268-272.
doi pubmed - Drugs@FDA: FDA approved drug products. Fda.gov. Published 2009. https://www.accessdata.fda.gov/scripts/cder/daf/.
- Rainwater-Lovett K, Redd JT, Stewart MA, Calles NE, Cluff T, Fang M, Panaggio MJ, et al. Real-world effect of monoclonal antibody treatment in COVID-19 patients in a diverse population in the United States. Open Forum Infect Dis. 2021;8(8):ofab398.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


