| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 1, January 2023, pages 19-24
Sarcoid-Like Granulomatosis of the Lung Related to Durvalumab After Chemoradiation for Pulmonary Squamous Cell Carcinoma
Seigo Minamia, Hironao Yasuokab, Nao Shoshiharaa, Daisuke Ishidac, Yasushi Sakamakic
aDepartments of Respiratory Medicine, Osaka Police Hospital, Osaka, Japan
bDepartments of Pathology, Osaka Police Hospital, Osaka, Japan
cDepartments of Respiratory Surgery, Osaka Police Hospital, Osaka, Japan
dCorresponding Author: Seigo Minami, Department of Respiratory Medicine, Osaka Police Hospital, Tennoji-ku, Osaka-City, Osaka 543-0035, Japan
Manuscript submitted December 14, 2022, accepted December 27, 2022, published online December 30, 2022
Short title: Sarcoid-Like Granulomatosis Due to Durvalumab
doi: https://doi.org/10.14740/jmc4038
| Abstract | ▴Top |
Sarcoid-like granulomatosis is a unique immune-related adverse event (irAE) in cancer patients treated with immune checkpoint inhibitors (ICIs). This irAE is infrequent, reported to range from 2% to 22.2% of melanoma treated with ICI. In a case of granulomatosis localized in the lung, it is difficult to differentiate granulomatosis from cancer progression or metastases. Herein, we report a case of ICI-induced sarcoid-like granulomatosis of the lung, which was confusable with localized recurrence of the primary lung cancer. A 56-year-old woman with c-stage IIIA of pulmonary squamous cell carcinoma in the right lower lobe received chemo-radiotherapy with two courses of cisplatin and vinorelbine and concurrent thoracic irradiation, followed by 1-year durvalumab consolidation therapy. The tumor in the right S6 grew and presented abnormal uptake by fluorodeoxyglucose positron emission tomography (FDG-PET), 1.5 years after durvalumab. Neither computed tomography (CT) nor FDG-PET found mediastinal and distant metastases. She underwent right lower lobe lobectomy. Histopathologically, the tumor and sampled lymph nodes contained no residue of carcinoma cells but presented diffuse epithelioid granuloma with infiltration of inflammatory cells, partial necrotic lesions and many multinucleated giant cells. In immunohistochemical stains, CD3+ and CD8+ T cells predominantly infiltrated, while there were few CD4+ T cells and a small number of CD20+ B cells. We followed her without steroid and other immunosuppressant drug. We should pay attention to the development of sarcoid-like granulomatosis as a rare irAE, which is difficult to be differentiated from cancer progression.
Keywords: Durvalumab; Sarcoid-like granulomatosis; Immune checkpoint inhibitor; Immune-related adverse event; Non-small cell lung cancer
| Introduction | ▴Top |
Immune checkpoint inhibitors (ICIs) have become indispensable in the treatment of various types of malignancies. Commercially available ICIs are antibodies targeting programmed cell death protein 1 (PD-1), the PD-1 ligand (PD-L1), or cytotoxic T-lymphocyte antigen 4 (CTLA-4). Durvalumab is an anti-PD-L1 antibody, which has presented a solid evidence in the phase III trial of 1-year consolidation therapy in patients with inoperable stage III non-small cell lung cancer (NSCLC) who did not have disease progression after induction platinum-based chemoradiotherapy [1].
A systemic granulomatous syndrome can be induced by several common drugs, which is clinically indistinguishable from sarcoidosis and is known as drug-induced sarcoidosis-like reaction. Most reported drugs included combination antiretroviral therapy, tumor necrosis factor alpha antagonist, interferon therapy and ICIs. Sarcoid-like granulomatosis is a unique immune-related adverse event (irAE) in cancer patients treated with ICIs. The frequency of granulomatous reactions ranged from 2% to 22.2% of melanoma ICI cases [2-4]. In speculation, this entity is due to uncontrolled T-helper 1-mediated immune responses, which produce granulomas with macrophages surrounded by an inflammatory ring with predominant CD4+ over CD8+ T lymphocytes [5].
We here report a patient with stage III NSCLC who presented sarcoid-like granulomatosis of the lung induced by durvalumab after concurrent chemo-radiotherapy.
| Case Report | ▴Top |
Investigations
A 56-year-old Japanese woman was referred to our department of respiratory medicine for intermittent bloody sputum and a tumor shadow in the right lower lobe in chest X-ray and computed tomography (CT) scan (Fig. 1a). She was a current smoker with 55.5 pack-year smoking history. Her medical history was only hyperlipidemia. The tumor was bronchoscopically visible at the right intermediate bronchus. She was diagnosed of squamous cell carcinoma by transbronchial biopsy (Fig. 2a). Tumor proportion score (TPS) of PD-L1 was low expression (TPS 30-40%) (22C3 pharmDx assay) (Fig. 2b). Whole body CT with contrast enhancement and fluorodeoxyglucose positron emission tomography (FDG-PET) revealed a 36 × 28 mm diameter tumor in the right lower lobe with a maximum standardized uptake value (SUVmax) of 6.3, and ipsilateral mediastinal lymph node metastases with SUVmax of 8.8. The cancer was classified as c-stage IIIA, c-T1cN2M0, in the seventh edition staging system of Union for International Cancer Control (UICC)-TNM classification. She received chemo-radiotherapy with two courses of cisplatin and vinorelbine and concurrent thoracic irradiation of once daily 2Gy × 33 fractions [6], followed by durvalumab consolidation therapy (at a dose of 10 mg/kg of body weight intravenously, every 2 weeks for up to 12 months). Persistent fever, slow moving and incoherent behavior occurred 5 to 6 months after durvalumab. She was diagnosed of an irAE of isolated adrenocorticotropic hormone (ACTH) deficiency. Supplemental hydrocortisone improved her symptoms.
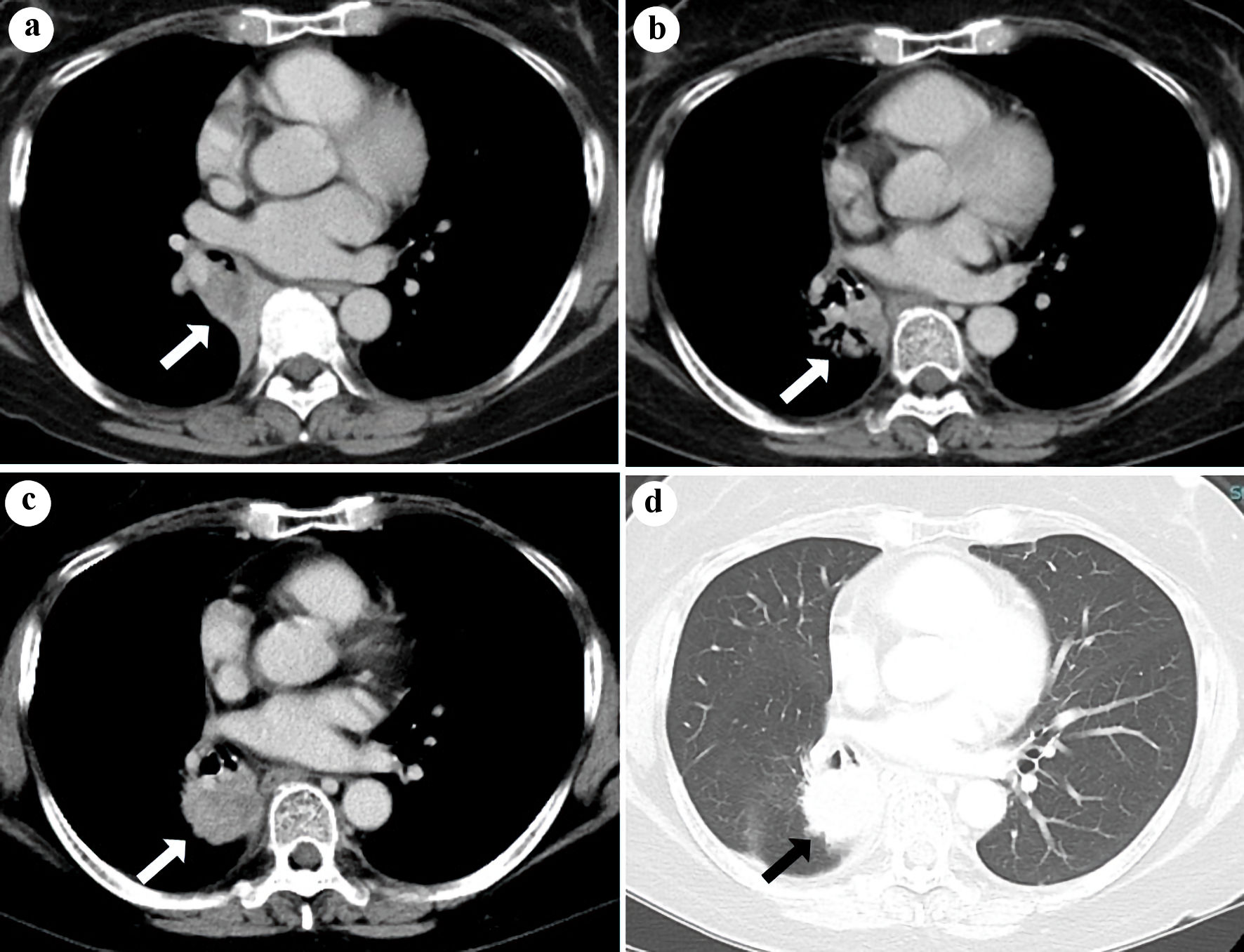 Click for large image | Figure 1. Chest CT (a) in October 2019, before chemo-radiation, (b) in January 2022, 1 year after durvalumab and (c, d) in July 2022, 1.5 years after durvalumab. The white (a-c) or black (d) arrows indicate the shadow of the primary tumor (a, b) or sarcoid-like granulomatosis (c, d) in the right lower lobe. CT: computed tomography. |
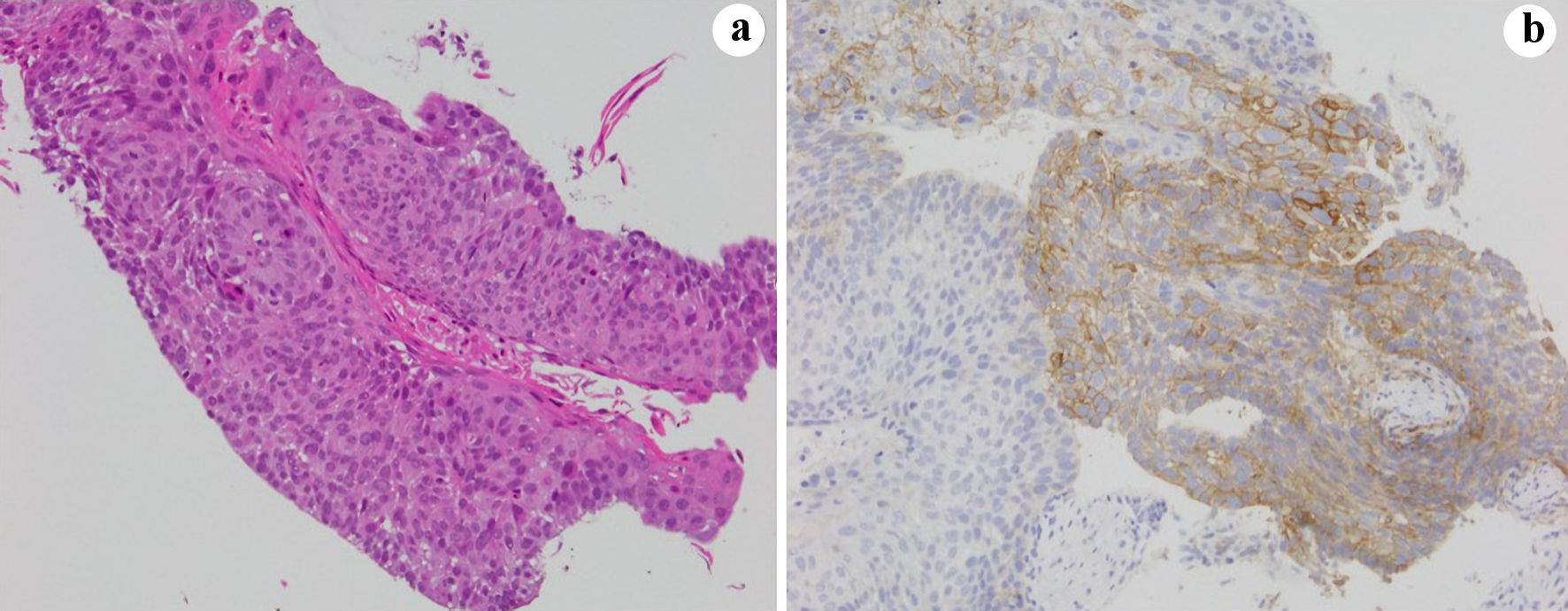 Click for large image | Figure 2. Bronchoscopic sample in October 2019 at the diagnosis. (a) Hematoxylin and eosin and (b) PD-L1 (22C3 pharmDx assay) stains. PD-L1: PD-1 ligand; PD-1: programmed cell death protein 1. |
Diagnosis
Thereafter, comparison of follow-up CTs of 1 and 1.5 years after durvalumab showed growth of the tumor in the right S6, which had lost intra-tumorous air bronchogram (Fig. 1b-d). PET-CT also revealed abnormal uptake in the tumor in the right S6 (SUVmax, 4.7) (Fig. 3). Neither CT nor FDG-PET found mediastinal and distant metastases (Fig. 1c-d, 3). Based on these images, we suspected loco-regional recurrence of her lung cancer. We did not try endobronchial ultrasound-guided transbronchial needle aspiration biopsy (EBUS-TBNB) at a risk of arterial vessels surrounding the tumor.
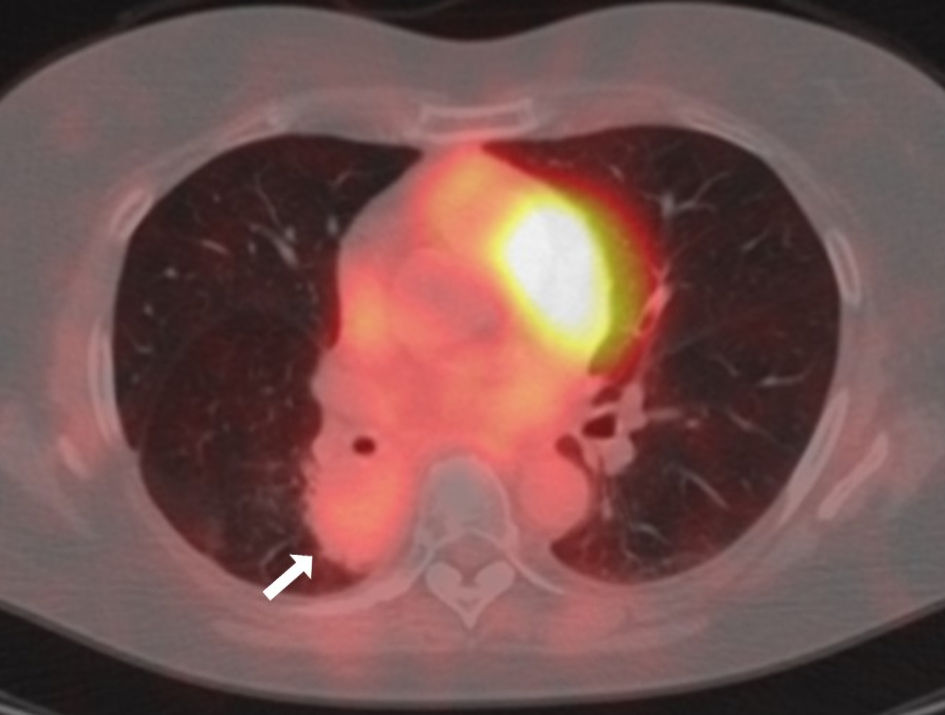 Click for large image | Figure 3. Fused image of fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) in July 2022 before the right lower lobe lobectomy. The white arrow indicates the sarcoid-like granulomatosis lesion. FDG-PET: fluorodeoxyglucose positron emission tomography; CT: computed tomography. |
Treatment
She underwent curative-intent right lower lobe lobectomy. The resected right lower lobe macroscopically contained a poorly-marginated and grayish white tumor (Fig. 4a). However, there was no residue of carcinoma cells in the resected tumor and sampled lymph nodes. Histopathologically, the tumor contained widespread organized lesions with infiltration of inflammatory cells, diffuse epithelioid granuloma with partial necrotic lesions, and many multinucleated giant cells (Fig. 4b, c). Immunohistochemical stains showed that CD3 (+) and CD8 (+) T cells predominantly infiltrated, but CD79α (+) B cells also appeared. There were few CD4 (+) T cells and a relatively small number of scattered CD20 (+) B cells (Fig. 5a-d). No organism was identified by Ziehl-Neelsen, periodic acid-Schiff and Grocotts stains. A serum T-SPOT TB test and anti-MAC antibody were negative, and angiotensin-converting enzyme were not elevated (11.2 U/L). Thus, we diagnosed her with sarcoid-like granulomatosis of the lung, which might have been induced by durvalumab. We did not administer steroid and other immunosuppressant drug.
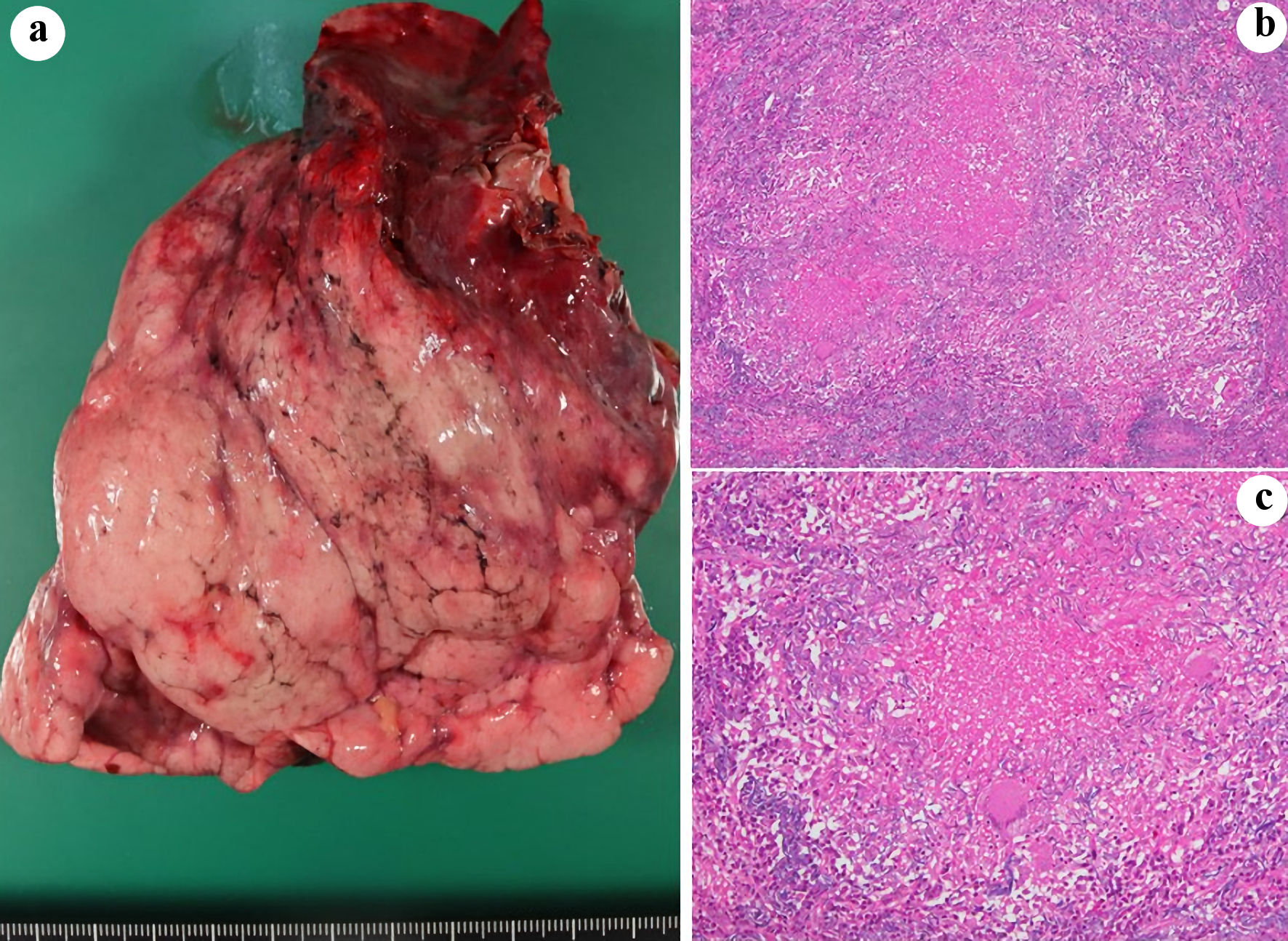 Click for large image | Figure 4. Macroscopical (a) and histopathological (b, c) findings of the resected right lower lobe. (b, c) Victoria blue/hematoxylin and eosin stain (magnification; (b) × 4, (c) × 10). |
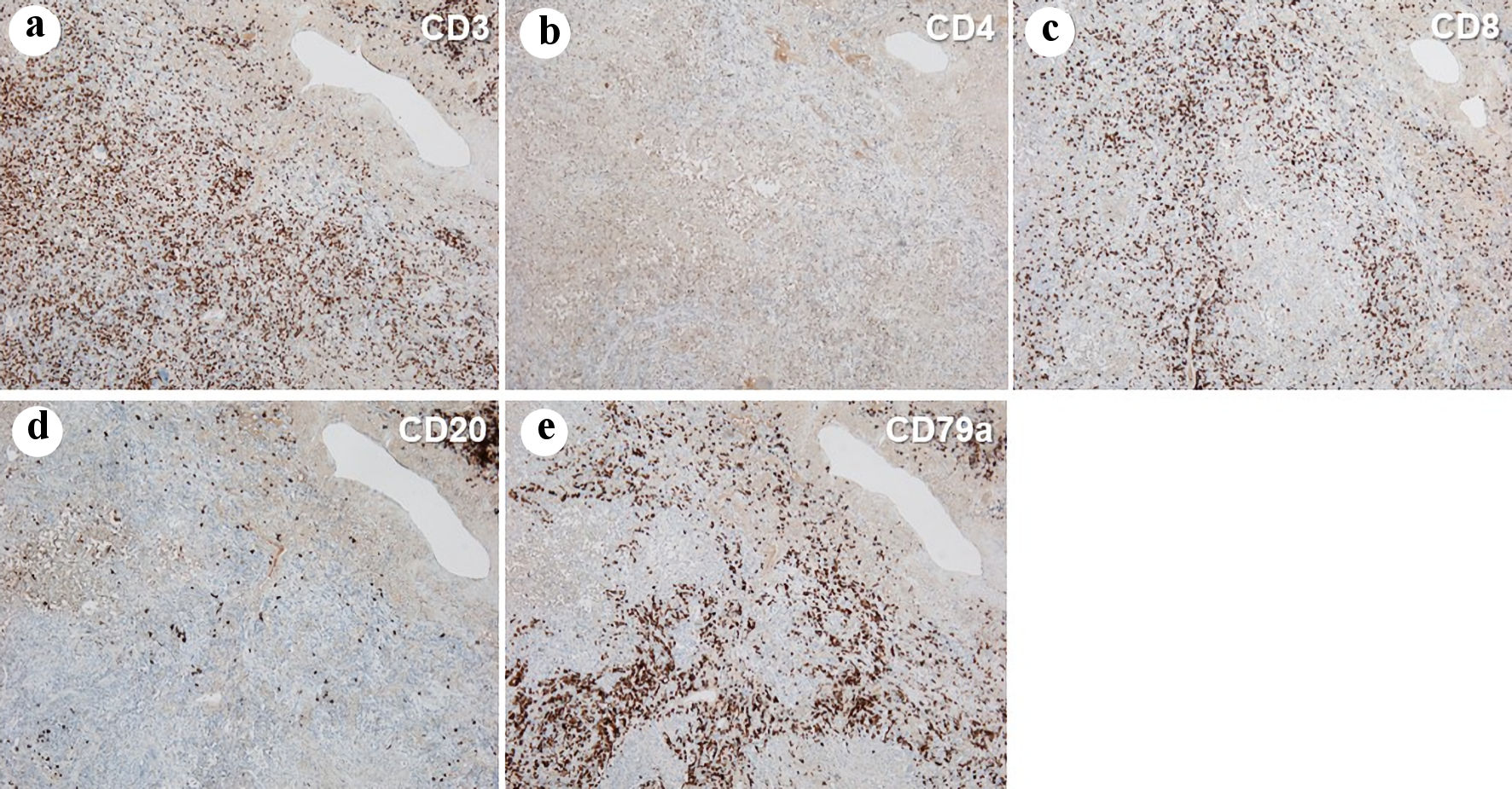 Click for large image | Figure 5. Immunohistochemistry of the resected right lower lobe, stained by anti-CD3 (a), CD4 (b), CD8 (c), CD20 (d) and CD79a (e) antibodies (magnification: × 4). |
Follow-up and outcomes
Neither feature of systemic sarcoidosis nor cancer relapse appeared 3 months after the surgery.
| Discussion | ▴Top |
This was the first case of durvalumab-induced sarcoid-like granulomatosis of the lung after concurrent chemo-radiotherapy for locally advanced NSCLC. Although sarcoid-like granulomatosis is an infrequent but possible irAE, our intrapulmonary granulomatosis was interesting in the following four untypical findings, which confusingly made our case a mimicker of cancer disease progression. First, the granulomatous consolidation emerged only in the primary tumor area, but not in the hilar and mediastinal lymph nodes. According to a French case-control study of 28 patients with sarcoid-like granulomatosis reaction associated with immunotherapy, mediastinal or peri-hilar thoracic lymph nodes was the most frequent sarcoidosis localization screened by CT scan in 92.9% of patients, while lung parenchymal involvement was found only in 50.0% of patients [7]. Another French retrospective study of 18 melanoma patients with histologically proven ICI-induced granulomatosis, chest CT scans revealed mediastinal adenopathies in all patients and interstitial involvement in nine patients [8]. Second, PET-CT showed FDG uptake in the granulomatous consolidation. Our SUVmax (4.7) in the granulomatous consolidation was lower than the optimal SUV cut-off value of 6.0 to 7.5 for differentiation of malignant or benign mediastinal lymphadenopathy. At these two points on the basis of comparisons of PET-CT and EBUS-TBNB, the sensitivity and specificity were 73% and 70% in a Pakistan study [9], and 78.5% and 81.2% in an Indian study [10], respectively. However, the optimal SUV cut-off value for diagnosing cancer tumor in the lung remains unknown. FDG uptake is sometimes confusing because its avidity can be noted in infectious and inflammatory lesions. Third, approximately 30 months after the initiation of 1-year durvalumab therapy, the granulomatous shadow appeared. In the French retrospective study at Gustave Roussy, the median periods from the ICI introduction to the clinical manifestations concomitantly with the radiological signs in 10 patients and to histological diagnosis in 13 patients were only 2 and 4 months, respectively [8]. In a recent systematic review of 91 patients with advanced melanoma and ICI-induced sarcoidosis-like reaction, the mean time from the ICI initiation until the onset of sarcoid-like reaction was only 7.1 months [11]. In the series of four cases of sarcoid-like granulomatosis of the lung as an irAE, the median time from the start of ICI to the onset of intrapulmonary shadows was 12.8 months (range from 8.0 to 20.7 months) [12]. Compared with the previous studies, our rare irAE was extremely late-onset. Finally, unlike the four case series by Nishino et al, the characteristic appearance of ground glass opacity halo surrounding a focal round consolidation was not clear in our case [12]. Pulmonary involvement may manifest with a wide range of radiographic findings including bilateral patchy ground-glass opacities, multifocal nodular changes, or focal consolidation with or without accompanying lymphadenopathy [13]. Although granulomatosis should be reminded as a differential diagnosis of a tumor progression or relapse during or after ICI therapy, it is difficult to differentiate granulomatosis from a tumor progression.
The most common methods of pathological confirmation of drug-induced sarcoidosis-like reaction is biopsy from skin and mediastinal lymph nodes by endobronchial ultrasound aspiration [14]. In the previous French study of 18 melanoma patients, histological confirmation of granuloma was obtained by biopsy of mediastinal nodes in 13 patients, liver in two, kidney in one, skin in one, and supraclavicular nodes in one [8]. It remains unknown whether the surgical resection is necessary to prevent a systemic manifestation in a case of localized sarcoid-like granulomatosis. Our case was insufficient to answer this question, partially because we could not deny the possibility of localized appearance of sarcoid-like reaction, which was unlikely to become systemic, and chiefly because we did not follow this patient for a long time. In any case of localized change of shadow image, we should not hesitate to obtain histological specimen in some appropriate way, including invasive surgery.
In conclusion, we should pay careful attention to the development of sarcoid-like granulomatosis as a rare irAE, which is difficult to be differentiated from cancer progression.
Learning points
The main take away points from this case report is that sarcoid-like granulomatosis is a rare irAE but is confusingly similar to cancer progression. We should be more conscious of and careful of the development of sarcoid-like granulomatosis as an infrequent but possible irAE. In a case of intrapulmonary localized change during or after immunotherapy, we should try to obtain histological specimen. The invasive surgery is sometimes necessary to diagnose this irAE.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
All authors have no conflict of interest to declare.
Informed Consent
Not applicable because the manuscript has been sufficiently deidentified to protect the patient.
Author Contributions
S. Minami and N. Shoshihara were involved in chemo-radiotherapy, immunotherapy and outpatient follow-up. D. Ishida and Y. Sakamaki performed the surgery. H. Yasuoka made pathological diagnosis. S. Minami drafted the report. All authors read and critically reviewed the manuscript, and then approved the final submitted version.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
ACTH: adrenocorticotropic hormone; CT: computed tomography; CTLA-4: cytotoxic T-lymphocyte antigen 4; EBUS-TBNB: endobronchial ultrasound-guided transbronchial needle aspiration biopsy; FDG-PET: fluorodeoxyglucose positron emission tomography; ICI: immune checkpoint inhibitor; irAE: immune-related adverse event; NSCLC: non-small cell lung cancer; PD-L1: programmed cell death protein 1; SUVmax: maximum standardized uptake value; TPS: tumor proportion score
| References | ▴Top |
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919-1929.
doi pubmed - Dimitriou F, Frauchiger AL, Urosevic-Maiwald M, Naegeli MC, Goldinger SM, Barysch M, Franzen D, et al. Sarcoid-like reactions in patients receiving modern melanoma treatment. Melanoma Res. 2018;28(3):230-236.
doi pubmed - Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse events associated with immune checkpoint blockade in patients with cancer: a systematic review of case reports. PLoS One. 2016;11(7):e0160221.
doi pubmed - Bronstein Y, Ng CS, Hwu P, Hwu WJ. Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy. AJR Am J Roentgenol. 2011;197(6):W992-W1000.
doi pubmed - Berthod G, Lazor R, Letovanec I, Romano E, Noirez L, Mazza Stalder J, Speiser DE, et al. Pulmonary sarcoid-like granulomatosis induced by ipilimumab. J Clin Oncol. 2012;30(17):e156-159.
doi pubmed - The Japan Lung Cancer Society: guidelines for diagnosis and treatment of lung cancer, 7th edition. Kanehara Publisher; 2022.
- Cabanie C, Ammari S, Hans S, Pobel C, Laparra A, Danlos FX, Chanson N, et al. Outcomes of patients with cancer and sarcoid-like granulomatosis associated with immune checkpoint inhibitors: A case-control study. Eur J Cancer. 2021;156:46-59.
doi pubmed - Melin A, Routier E, Roy S, Pradere P, Le Pavec J, Pierre T, Chanson N, et al. Sarcoid-like granulomatosis associated with immune checkpoint inhibitors in melanoma. Cancers (Basel). 2022;14(12):2937.
doi pubmed - Khalid U, Akram MJ, Abu Bakar M, Butt FM, Ashraf MB. Elucidating the etiologies of 18f-fluorodeoxyglucose-avid mediastinal lymph nodes among cancer patients in a tuberculosis-endemic region using endobronchial ultrasound. Cureus. 2021;13(11):e19339.
doi - Luthra K, Singh J. Evaluation of 18FDG PET-CT-positive mediastinal-hilar lymph nodes in extrathoracic malignancies by EBUS-TBNA; correlation of SUVmax, and short-axis diameter with the final diagnosis. Indian J Cancer. 2021.
doi pubmed - Rubio-Rivas M, Moreira C, Marcoval J. Sarcoidosis related to checkpoint and BRAF/MEK inhibitors in melanoma. Autoimmun Rev. 2020;19(8):102587.
doi pubmed - Nishino M, Sholl LM, Awad MM, Hatabu H, Armand P, Hodi FS. Sarcoid-like granulomatosis of the lung related to immune-checkpoint inhibitors: distinct clinical and imaging features of a unique immune-related adverse event. Cancer Immunol Res. 2018;6(6):630-635.
doi pubmed - Rambhia PH, Reichert B, Scott JF, Feneran AN, Kazakov JA, Honda K, Koon H, et al. Immune checkpoint inhibitor-induced sarcoidosis-like granulomas. Int J Clin Oncol. 2019;24(10):1171-1181.
doi pubmed - Chopra A, Nautiyal A, Kalkanis A, Judson MA. Drug-induced sarcoidosis-like reactions. Chest. 2018;154(3):664-677.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


