| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 7, July 2023, pages 227-231
Intravenous Immunoglobulin-Associated Severe Hemolytic Anemia
Ojbindra KCa, c , Ananta Subedib, Rakshya Sharmab
aDepartment of Hospital Medicine, Faith Regional Health Services, Norfolk, NE, USA
bDepartment of Hospital Medicine, Avera McKennan Hospital and University Health Center, Sioux Falls, SD, USA
cCorresponding Author: Ojbindra KC, Department of Hospital Medicine, Faith Regional Health Services, Norfolk, NE, USA
Manuscript submitted June 9, 2023, accepted June 26, 2023, published online July 12, 2023
Short title: IVIG-Induced Hemolytic Anemia
doi: https://doi.org/10.14740/jmc4126
| Abstract | ▴Top |
Intravenous immunoglobulin (IVIG) is used to treat immunodeficiency conditions, neuro-immunological, infection-related, autoimmune, and inflammatory disorders and is typically well tolerated. A hematological adverse reaction such as hemolytic anemia and neutropenia is known to occur with IVIG, which is usually transient and subclinical. However, severe hemolytic anemia is known to occur in some cases. We present a case of a 66-year-old man who developed severe symptomatic hemolytic anemia after receiving IVIG for acute inflammatory demyelinating polyneuropathy (AIDP). The patient had known risk factors such as non-O blood group, high cumulative dose of IVIG, and underlying autoimmune condition, which would have put him at high risk for developing hemolytic anemia after IVIG. Therefore, it is prudent for clinicians to have increased awareness regarding the potential for severe hemolysis and closely monitor these patients with risk factors after treatments to identify this adverse reaction before more severe complications occur.
Keywords: Hemolytic anemia; Intravenous immunoglobulin; Isoagglutinins
| Introduction | ▴Top |
Intravenous immunoglobulin (IVIG) is produced from pooled human plasma donated by several thousand screened donors. The immune globulin-containing fraction is isolated from the plasma through cold alcohol fractionation. After undergoing various treatments, such as filtration, refinement, and viral inactivation processes, IVIG is produced [1].
IVIG can be used to treat primary and secondary immunodeficiency conditions, neuro-immunological disorders such as acute inflammatory demyelinating polyneuropathy (AIDP), chronic inflammatory demyelinating polyneuropathy (CIDP), autoimmune conditions like immune thrombocytopenia (ITP), and infections such as toxic shock syndrome (TSS) [2]. IVIG is typically well tolerated; most adverse reactions, such as flu-like symptoms, including headaches, chills, fatigue, and myalgia, are mild and transient. However, in approximately 2-6% of patients, potentially severe adverse reactions such as thromboembolic complications, acute kidney injury (AKI), hyponatremia, aseptic meningitis, and hemolytic anemia have been reported [3-9]. Hemolysis is a potential adverse effect related to IVIG administration that occurs in approximately 1.6% of patients, which is usually subclinical and mostly not treated [4].
Here, we discuss a case of a patient who experienced severe hemolytic anemia after receiving IVIG treatment and was treated with steroids with improvement.
| Case Report | ▴Top |
Investigations
A 66-year-old male with no significant past medical history presented to a primary physician’s office with complaints of numbness and tingling in his bilateral lower extremities of a 2-week duration, which gradually ascended to his calves and bilateral hands. The patient underwent a workup, including cervical spine and lumbar spine magnetic resonance imaging (MRI), which did not reveal any significant findings. Blood workup for neuropathy, such as thyroid-stimulating hormone (TSH), vitamin B12, homocysteine, methylmalonic acid, hemoglobin A1c (HbA1c), and human immunodeficiency virus (HIV), were done, which were unremarkable. The patient was then referred to a neurology clinic, where electromyography (EMG) and a nerve conduction study (NCS) were performed, which showed findings consistent with AIDP, a variant of Guillain-Barre syndrome due to absence of motor symptoms. After confirming that the patient did not have IgA deficiency, IVIG was started at a dosage of 0.4 g/kg for 5 days. The patient noted improvement in foot numbness and paresthesia 2 days after infusion. However, after 5 days, the patient started to have shortness of breath and feeling lethargic and generalized weakness. He was referred to our center for further evaluation and management. The patient’s vitals were stable on presentation to our center, and the physical examination was unremarkable.
Diagnosis
His chest X-ray was unremarkable. D-dimer, B-type natriuretic peptide (BNP), and troponin were within normal limits and electrocardiogram revealed normal sinus rhythm which ruled out pulmonary and cardiac causes of shortness of breath. A blood workup revealed a drop in hemoglobin (Hb) levels from 13.5 to 8.3 g/dL within 3 days. He reported no melena, hematemesis, hemoptysis, or trauma resulting in acute blood loss. His fecal occult blood test was negative. The decrease in Hb levels raised concerns for hemolytic anemia.
Further workup to investigate hemolysis included an elevated reticulocyte count of 13% (normal range 0.5-1.5%), elevated bilirubin at 3.5 mg/dL, lactate dehydrogenase (LDH) at 322 U/L, low haptoglobin at < 30 mg/dL, a positive direct antiglobulin testing (DAT) with IgG+, and negative complement. A peripheral blood smear showed normochromic normocytic anemia with 3+ spherocytes (Fig. 1). The bone marrow biopsy revealed a hypercellular bone marrow with erythroid hyperplasia and negative flow cytometry (Fig. 2). Given recent use of IVIG for AIDP, he was diagnosed with IVIG therapy-induced hemolytic anemia.
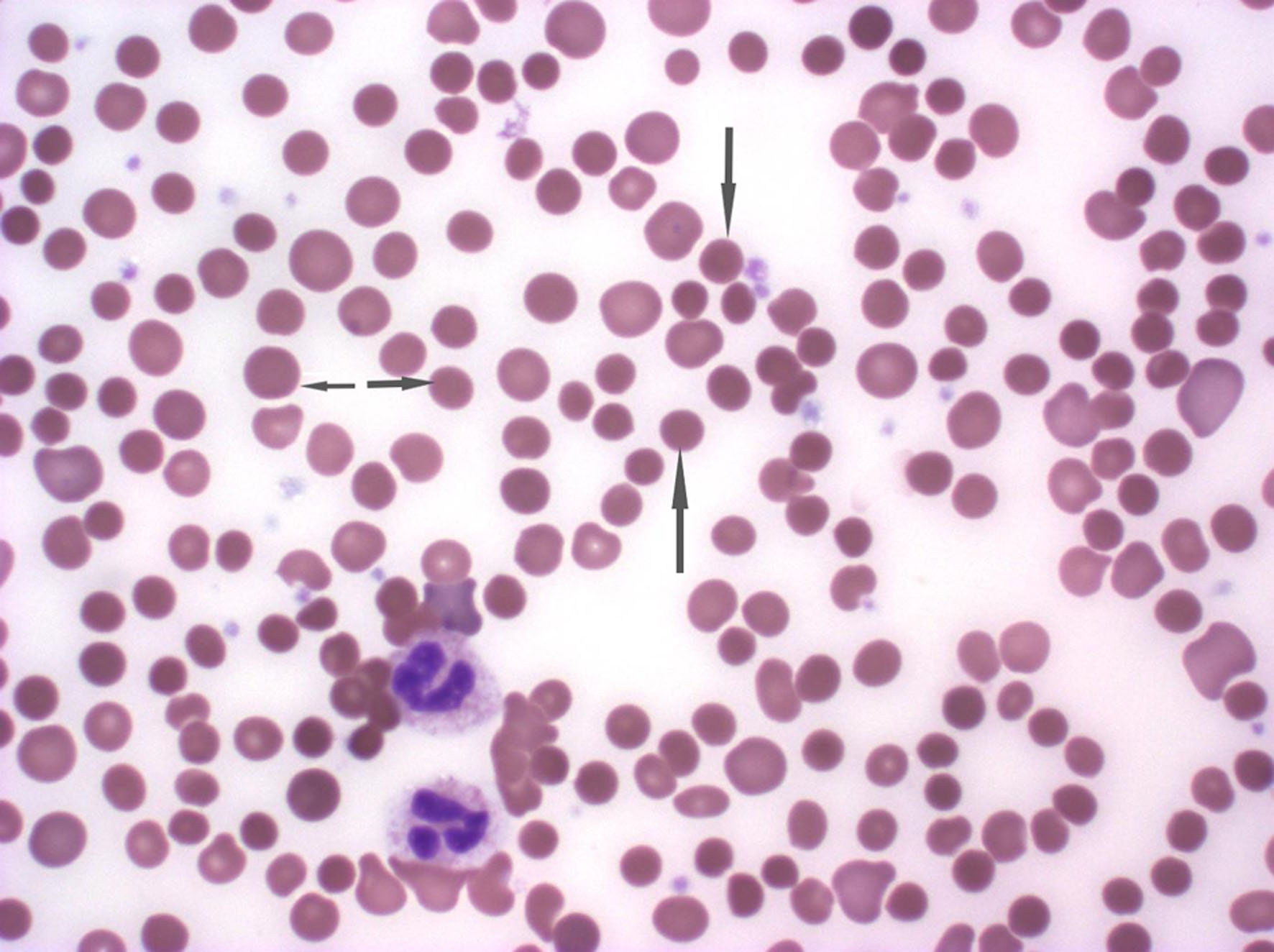 Click for large image | Figure 1. The peripheral smear demonstrating normocytic normochromic anemia with 3+ spherocytes. The arrowhead demonstrates the spherocytes. |
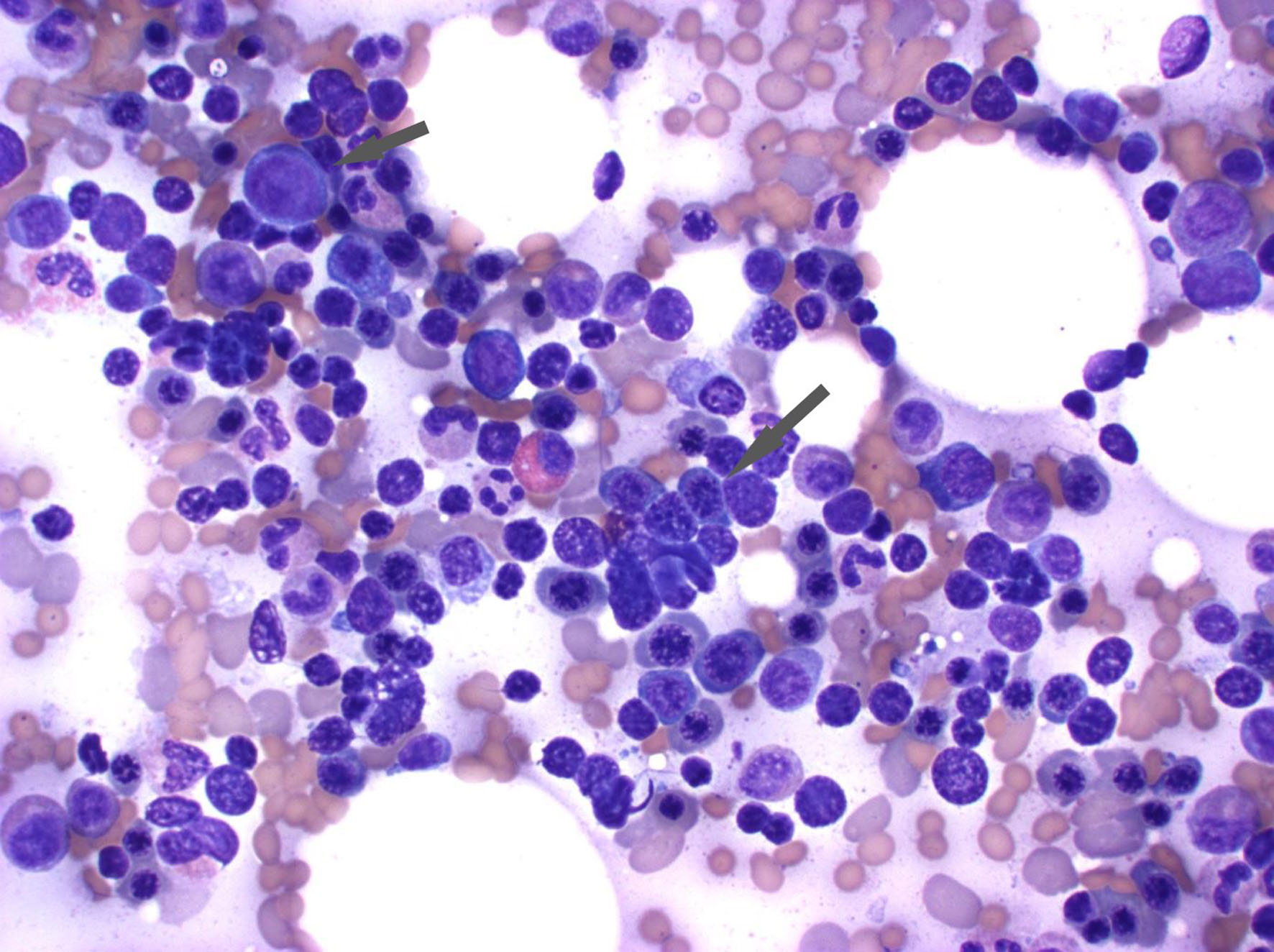 Click for large image | Figure 2. The bone marrow aspirate reveals erythroid hyperplasia. The arrowhead demonstrates the erythroid precursor cells with round dark nuclei without much cytoplasm. |
Treatment
The patient was started on prednisone at a dosage of 1 mg/kg, which was continued for 4 days. His symptoms improved, and he was subsequently discharged on a maintenance dose of prednisone at 100 mg daily. He was also discharged on folic acid and trimethoprim-sulfamethoxazole.
Follow-up and outcomes
On follow-up, the patient’s Hb level improved to 12.9 g/dL, and his haptoglobin level increased to 188 mg/dL (normal range: 44 - 215 mg/dL). His LDH level also improved to 174 U/L (normal range: 140 - 271 U/L). The patient’s prednisone dose was gradually tapered by decreasing 10 mg every week for the next 2 months on an outpatient basis. The Hb level and other markers of hemolysis (reticulocytes count, haptoglobin, LDH, and bilirubin) gradually improved during hospitalization and follow-up, as demonstrated in Table 1.
 Click to view | Table 1. Trend of Hemoglobin and Hemolysis Markers During Hospitalization and Follow-Up |
| Discussion | ▴Top |
Our case was a 66-year-old man who weighed 91 kg and was diagnosed with the autoimmune condition AIDP and was given 0.4 g/kg IVIG for a total of 5 days, which amounted to a cumulative dose of 182 g. The blood group of the patient was AB positive. He developed severe hemolytic anemia after 5 days of the last dose of IVIG, 10 days from the initial first dose of IVIG. Our patient had known risk factors such as non-O blood group, high cumulative dose of IVIG, and underlying autoimmune condition, which would have put him at high risk for developing hemolytic anemia after IVIG. Therefore, it would have been prudent if clinicians had performed hemolytic screening after infusion of IVIG in this case before he developed symptomatic hemolytic anemia.
The use of IVIG can lead to hematologic adverse effects such as neutropenia and hemolysis, which are usually transient [4]. However, clinically significant severe hemolytic anemia has been reported with IVIG use [6, 8, 10, 11]. The incidence of hemolysis has been reported to be around 1.6% in patients receiving IVIG [12]. The passive transfer of antibodies present in IVIG reacts with red blood cell (RBC) antigen predominantly ABO blood group system (anti-A and anti-B), which is the proposed mechanism of hemolysis with IVIG use. The risk of hemolysis is higher in patients with non-O blood group type. O is the most predominant blood group type; it is assumed that in the plasma product, a significant amount of antibodies specific to blood groups A and B (anti-A and anti-B isoagglutinin) are present. These isoagglutinins get transferred and react with the RBC antigens, thus causing hemolysis [13]. The highest risk of IVIG-associated hemolysis has been seen in blood group AB followed by A, most likely because there are higher levels of anti-A than anti-B in plasma [14]. Anti-A titers greater than 1:16 are more likely to cause significant hemolysis [12].
A study conducted at the Ottawa Hospital found that out of 1,000 patients who received IVIG, 16 of them had hemolysis, 15 out of 16 cases of hemolysis had high cumulative doses of IVIG, non-O blood group, and positive inflammatory serological markers, while 10 out of 16 cases were female [12]. Patients receiving high doses of IVIG (1 to 2 g/kg/day) and cumulative doses (> 100 g) are seen to have more incidences of hemolysis as higher doses of IVIG will have higher doses of antibodies. Female sex and increased inflammatory markers such as erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and ferritin have been associated with increased risk factors for hemolysis [12, 15]. The hemolysis has been reported to occur from 12 h to 10 days after the first IVIG infusion [4, 15]. The clinical symptoms of hemolysis can be related to anemia, or patients may be asymptomatic and only diagnosed with low Hb levels during a clinical examination. If hemolysis is suspected, a hemolytic workup should be performed, including tests such as Hb, total bilirubin, reticulocyte count, LDH, haptoglobin, and peripheral blood smear. It is recommended to perform hemolytic screening 5 - 7 days after infusion in patients receiving higher doses of IVIG and patients with non-O blood group [15].
The hemolysis is due to the passive transfer of antibodies/isoagglutinin in IVIG and occurs with higher doses of IVIG. For patients with clinically severe hemolysis and positive anti-A and anti-B antibody due to IVIG, it is advised to avoid re-administering the same product with same lot number. However, switching brands of IVIG that has lower titer of isoagglutinins may be helpful [11]. Subcutaneous immune globulin (SGIG) may be used as it has been rarely associated with hemolysis [11]. If transfusion is required, it is recommended to transfuse group O RBCs, to avoid the possibility of hemolysis [16]. If IVIG is to be avoided, the patient with AIDP should be treated with plasma exchange.
IVIG preparations with high anti-A/anti-B titers are associated with an increased incidence of hemolysis. IVIG manufacturing processes are based on combinations of cold ethanol precipitation to separate IgG from albumin followed by precipitation and chromatographic steps to increase IgG purity. The risk of hemolytic anemia is much higher with IVIG products, that have skipped the separation of FIII of the original ethanol fractionation process without another isoagglutinin reduction step [17, 18]. IVIG products that have utilized further purification processes such as immunoaffinity chromatography (IAC) have demonstrated reduced rate of hemolysis [14, 19].
Autoimmune hemolytic anemia is hemolysis caused by autoantibodies which could be warm agglutin-induced (antibody that is active at normal body temperature), cold agglutin-induced (antibody that is active below core body temperature) and drug-induced (antibody developed due to drugs) [20, 21]. IVIG induced hemolytic anemia not due to autoantibodies; it is due to passive transfer to antibodies/isoagglutinin in IVIG. However, steroid may play a role in downregulation of complement production and decrease the hemolysis in IVIG-associated hemolysis, and it may also decrease the underlying inflammatory state [22].
Conclusion
Hemolysis is a potential adverse reaction that is typically mild and transient after IVIG therapy. However, severe hemolysis with symptomatic anemia may occur in certain patients. Therefore, it is prudent for clinicians to have increased awareness regarding the potential for severe hemolysis and closely monitor these patients with risk factors after treatments to identify this adverse reaction before more severe complications occur.
Learning points
IVIG therapy can lead to severe hemolytic anemia in certain patients with risk factors for hemolysis. Clinicians should be aware of IVIG therapy’s potential severe adverse effects and monitor these patients closely, which can prevent associated morbidity and mortality.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Consent for publication of individual healthcare data and images was obtained from the patient presented in the case report.
Author Contributions
OKC: conceptualization, data collection, writing initial manuscript draft, writing and editing final manuscript. AS: patient care, data collection and writing initial manuscript draft. RS: patient care, review and editing of final manuscript.
Data Availability
The data supporting the finding of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Barahona Afonso AF, Joao CM. The production processes and biological effects of intravenous immunoglobulin. Biomolecules. 2016;6(1):15.
doi pubmed pmc - Brand A, De Angelis V, Vuk T, Garraud O, Lozano M, Politis D, European Mediterranean Initiative for Transfusion M. Review of indications for immunoglobulin (IG) use: Narrowing the gap between supply and demand. Transfus Clin Biol. 2021;28(1):96-122.
doi pubmed - Bilal J, Riaz IB, Hill JL, Zangeneh TT. Intravenous Immunoglobulin-Induced Pulmonary Embolism: It Is Time to Act! Am J Ther. 2016;23(4):e1074-1077.
doi pubmed - Guo Y, Tian X, Wang X, Xiao Z. Adverse effects of immunoglobulin therapy. Front Immunol. 2018;9:1299.
doi pubmed pmc - Perez EE, Orange JS, Bonilla F, Chinen J, Chinn IK, Dorsey M, El-Gamal Y, et al. Update on the use of immunoglobulin in human disease: A review of evidence. J Allergy Clin Immunol. 2017;139(3S):S1-S46.
doi pubmed - Pierce LR, Jain N. Risks associated with the use of intravenous immunoglobulin. Transfus Med Rev. 2003;17(4):241-251.
doi pubmed - Sekul EA, Cupler EJ, Dalakas MC. Aseptic meningitis associated with high-dose intravenous immunoglobulin therapy: frequency and risk factors. Ann Intern Med. 1994;121(4):259-262.
doi pubmed - Nguyen MK, Rastogi A, Kurtz I. True hyponatremia secondary to intravenous immunoglobulin. Clin Exp Nephrol. 2006;10(2):124-126.
doi pubmed - Welles CC, Tambra S, Lafayette RA. Hemoglobinuria and acute kidney injury requiring hemodialysis following intravenous immunoglobulin infusion. Am J Kidney Dis. 2010;55(1):148-151.
doi pubmed - Copelan EA, Strohm PL, Kennedy MS, Tutschka PJ. Hemolysis following intravenous immune globulin therapy. Transfusion. 1986;26(5):410-412.
doi pubmed - Quinti I, Pulvirenti F, Milito C, Granata G, Giovannetti G, La Marra F, Pesce AM, et al. Hemolysis in patients with antibody deficiencies on immunoglobulin replacement treatment. Transfusion. 2015;55(5):1067-1074.
doi pubmed - Daw Z, Padmore R, Neurath D, Cober N, Tokessy M, Desjardins D, Olberg B, et al. Hemolytic transfusion reactions after administration of intravenous immune (gamma) globulin: a case series analysis. Transfusion. 2008;48(8):1598-1601.
doi pubmed - Bonilla FA. Adverse effects of immunoglobulin G therapy: thromboembolism and haemolysis. Clin Exp Immunol. 2014;178(Suppl 1):72-74.
doi pubmed pmc - Hoefferer L, Glauser I, Gaida A, Willimann K, Marques Antunes A, Siani B, Wymann S, et al. Isoagglutinin reduction by a dedicated immunoaffinity chromatography step in the manufacturing process of human immunoglobulin products. Transfusion. 2015;55(Suppl 2):S117-121.
doi pubmed - Pendergrast J, Armali C, Callum J, Cserti-Gazdewich C, Jiwajee A, Lieberman L, Lau W, et al. A prospective observational study of the incidence, natural history, and risk factors for intravenous immunoglobulin-mediated hemolysis. Transfusion. 2021;61(4):1053-1063.
doi pubmed - Pintova S, Bhardwaj AS, Aledort LM. IVIG—a hemolytic culprit. N Engl J Med. 2012;367(10):974-976.
doi pubmed - Bellac CL, Hottiger T, Jutzi MP, Bogli-Stuber K, Sanger M, Hanschmann KM, Keller-Stanislawski B, et al. The role of isoagglutinins in intravenous immunoglobulin-related hemolysis. Transfusion. 2015;55(Suppl 2):S13-22.
doi pubmed - Cuesta H, El Menyawi I, Hubsch A, Hoefferer L, Mielke O, Gabriel S, Shebl A. Incidence and risk factors for intravenous immunoglobulin-related hemolysis: A systematic review of clinical trial and real-world populations. Transfusion. 2022;62(9):1894-1907.
doi pubmed pmc - Shebl A, Gabriel S, Van Dinther K, Hubsch A, Lawo JP, Hoefferer L, Welsh S. Isoagglutinin reduction in intravenous immunoglobulin (IgPro10, Privigen) by specific immunoaffinity chromatography reduces its reporting rates of hemolytic reactions: an analysis of spontaneous adverse event reports. Transfusion. 2020;60(6):1278-1286.
doi pubmed pmc - Jager U, Barcellini W, Broome CM, Gertz MA, Hill A, Hill QA, Jilma B, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: Recommendations from the First International Consensus Meeting. Blood Rev. 2020;41:100648.
doi pubmed - Garratty G, Petz LD. Drug-induced immune hemolytic anemia. Am J Med. 1975;58(3):398-407.
doi pubmed - Mohamed M. Intravenous immunoglobulin-associated hemolysis: risk factors, challenges, and solutions. Int J Clin Transfus Med. 2016;4:121-131.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


