| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 4, April 2023, pages 111-117
Primary Squamous Cell Lung Cancer With Frequent Episodes of Sustained Ventricular Tachycardia due to Myocardial Metastasis
Sayaka Toyoshia, Norihiko Funaguchia, c, Hirotoshi Ishigakia, Komei Yanaseb
aDepartment of Respiratory Medicine, Asahi University Hospital, Gifu 500-8523, Japan
bDepartment of Cardiology and Respiratory Medicine, Gifu University Graduate School of Medicine, Gifu 501-1194, Japan
cCorresponding Author: Norihiko Funaguchi, Department of Respiratory Medicine, Asahi University Hospital, Gifu 500-8523, Japan
Manuscript submitted February 23, 2023, accepted April 8, 2023, published online April 30, 2023
Short title: VT due to Myocardial Metastasis of Lung SCC
doi: https://doi.org/10.14740/jmc4066
| Abstract | ▴Top |
Myocardial metastasis from lung cancer rarely occurs. We encountered a patient with squamous cell lung cancer who was diagnosed with myocardial metastasis before death and sustained ventricular tachycardia during the course of the disease. The patient was a 56-year-old woman. A tumor was noted in the apex area of the left lung and was diagnosed as stage IVA of squamous cell lung cancer after a detailed examination. She underwent concurrent chemoradiotherapy with weekly treatment of carboplatin + paclitaxel. A 12-lead electrocardiogram performed upon admission for additional chemotherapy showed negative T waves in leads III, aVF, and V1-4. Transthoracic echocardiography and computed tomography showed a tumor lesion in the right ventricular wall, which was diagnosed as myocardial metastasis from lung cancer. During the course of the disease, the patient had frequent episodes of sustained ventricular tachycardia, which were refractory to treatment with antiarrhythmic drugs. However, the sinus rhythm was restored with cardioversion. Subsequently, the patient received palliative treatment and eventually died 4 months after the diagnosis of cardiac metastasis and 3 weeks after the diagnosis of ventricular tachycardia. Myocardial metastasis might reflect poor prognosis due to serious arrhythmia or some other complications. Therefore, the early diagnosis and appropriate treatment of cardiac metastasis by chemotherapy, cardiac radiotherapy, or surgery, are necessary prior to the development of symptoms in tolerant cases.
Keywords: Squamous cell lung cancer; Myocardial metastasis; Sustained ventricular tachycardia; Cardioversion
| Introduction | ▴Top |
Metastatic cardiac tumors are commonly found in 7.1-9.1% of autopsied patients with cancer and 2.3-18.3% of all autopsied patients [1, 2]. It is common to find metastasis of lung cancer in hilar and mediastinal lymph nodes, lungs, liver, bones, and brain. Although lung cancer often leads to distant metastasis, myocardial metastasis is rarely diagnosed before death. Since most cardiac metastases appear in patients with advanced stages of the disease, limited treatment options lead to a poor prognosis. Metastatic cardiac tumors can cause heart failure, ventricular or supraventricular arrhythmia, conduction disorders, syncope, embolism, and pericardial effusion. Rare cases of ventricular tachycardia (VT) due to metastatic cardiac tumors have been reported [3, 4]. Not infrequently, metastatic cardiac tumor invasion becomes a cause of death in affected patient.
We here report a patient who was diagnosed with myocardial metastasis, which was evidenced by an abnormal electrocardiogram (ECG) during the treatment of advanced squamous cell lung cancer and frequent episodes of sustained VT during the disease course and was difficult to treat.
| Case Report | ▴Top |
Investigations
The patient was a 56-year-old Japanese woman. She had a 36-year smoking history, smoking 20 cigarettes per day. In May 2018, the patient experienced left back pain. As there was no improvement in the pain intensity, she decided to visit our hospital to undergo consultation. Chest computed tomography (CT) showed a tumor in the S1+2 apex area of the left upper lobe. The tumor was diagnosed as squamous cell lung cancer by transbronchial biopsy. After detailed examination, the cancer was classified as c-T3N1M1b, stage IVA in the eighth edition staging system of Union for International Cancer Control (UICC-version 8)-TNM classification. The tumor was negative for both epidermal growth factor receptor (EGFR) gene mutation and anaplastic lymphoma kinase (ALK) fusion gene, and the tumor proportion score (TPS) of programmed cell death protein-1 ligand 1 (PD-L1) was high expression (TPS 70%) (22C3 pharmDx assay). Due to the occurrence of severe left back pain and invasion of tumor in the chest wall at the apex of the lung, local treatment was prioritized, although the tumor was at stage IVA. The patient received thoracic radiotherapy (60 Gy in 30 fractions at 2 Gy per fraction) concurrent with weekly carboplatin (area under the plasma concentration-time curve (AUC): 2) plus paclitaxel (40 mg/m2) for 6 weeks. The patient also received radiotherapy for right femoral metastasis. Denosumab was administered as treatment for bone metastasis, and oxycodone was administered as treatment for left back pain.
Diagnosis
In August of the same year, she was admitted to undergo chemotherapy. On physical examination, her temperature was 36.6 °C, heart rate was 70 beats per minute (bpm), blood pressure was 110/58 mm Hg, respiratory rate was 12 breaths per minute, and oxygen saturation was 97% on room air. No lymphadenopathy was noted. On auscultation, her lung sounds were clear, and her heart sounds were normal with no murmurs. Results of the laboratory test on admission showed mild anemia (hemoglobin: 10.9 g/dL). Biochemical tests showed no abnormal values. With regard to the tumor markers, the carcinoembryonic antigen (CEA) level (37.9 ng/dL) and cytokeratin fragment (CYFRA) level (9.2 ng/dL) were relatively high. Chest X-ray images on admission showed the presence of a tumor in the left upper lung field. Contrast-enhanced CT of the chest (Fig. 1) showed a 65 × 45 mm lesion with a heterogeneous contrast effect in S1+2 in the left upper lobe. It also showed tumor invasion in the chest wall. A 12-lead ECG showed poor R wave progression in leads V1-3, negative T waves in leads III, aVF, and V1-4 (Fig. 2a). Transthoracic echocardiography (TTE) showed a hypoechoic tumor in the right ventricular cavity (Fig. 3a). In addition, transesophageal echocardiography (TEE) showed normal wall motion, no valvular disease, and no pericardial effusion.
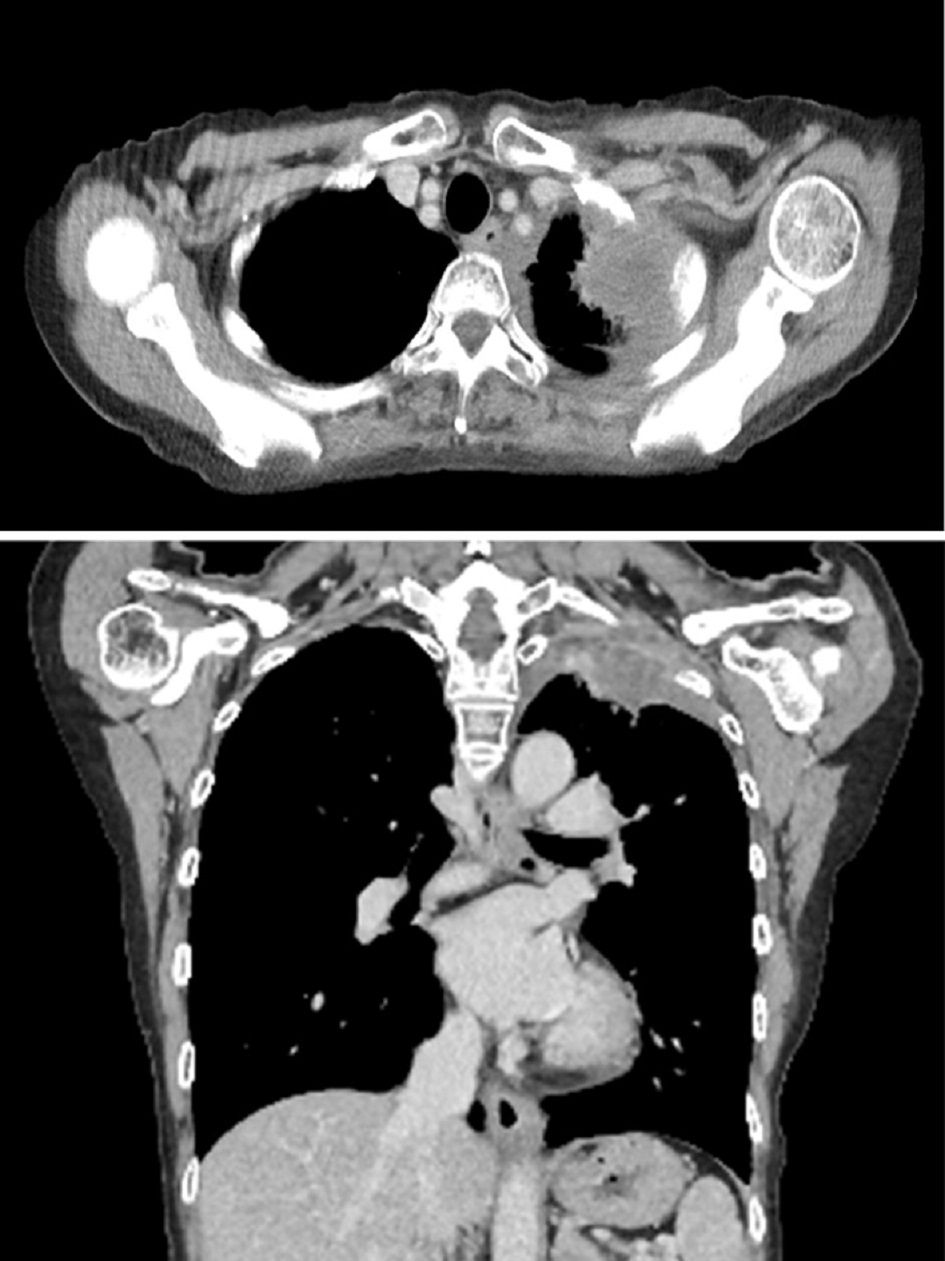 Click for large image | Figure 1. Contrast-enhanced computed tomography of the chest on admission. A 65 × 45 mm lesion with a heterogeneous contrast effect in S1+2 in the left upper lobe was observed. Tumor invasion was observed in the chest wall. |
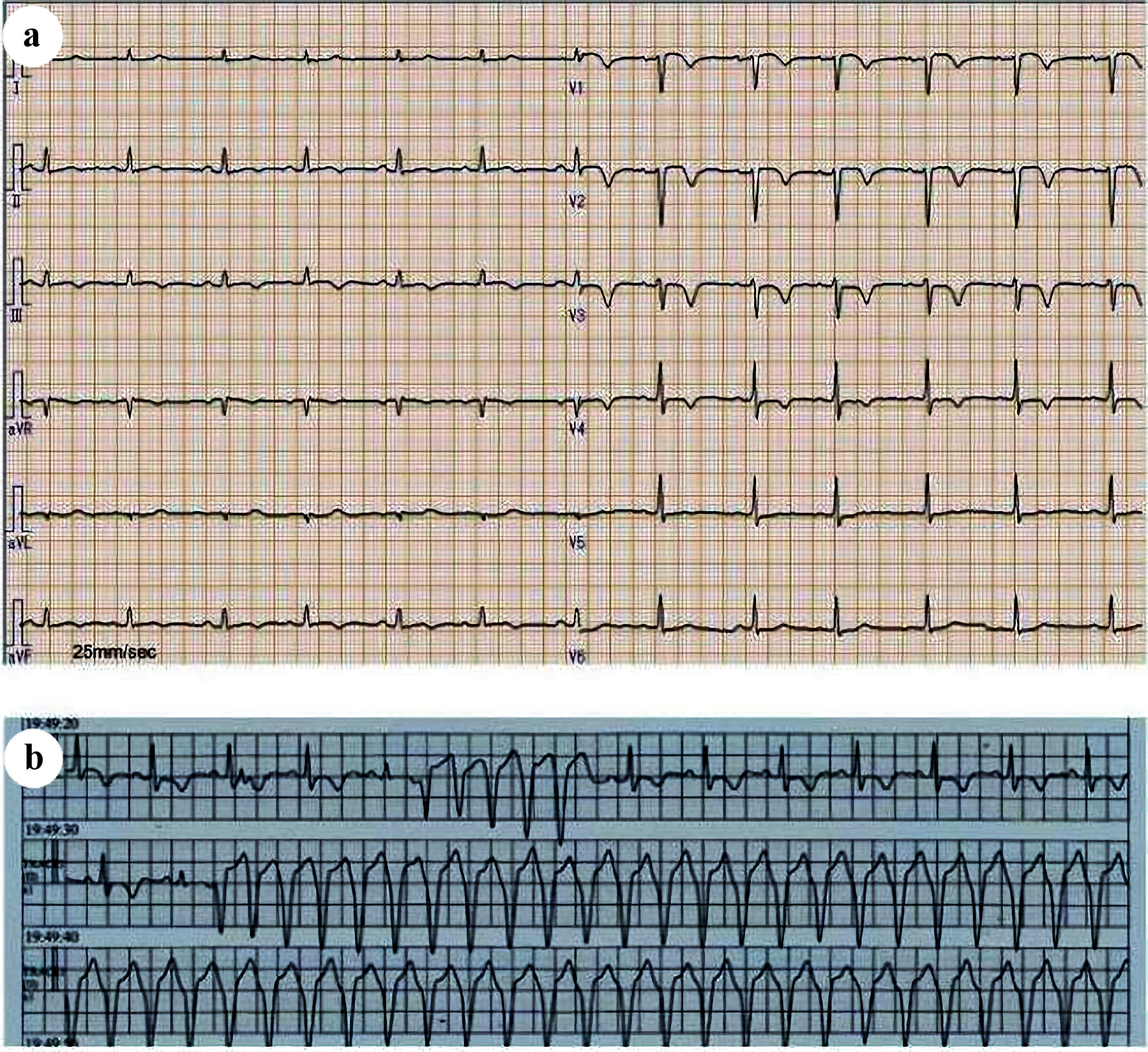 Click for large image | Figure 2. (a) The 12-lead electrocardiogram on admission. Poor R wave progression was observed in leads V1-3, and negative T waves in leads III, aVF, V1-4. (b) Electrocardiographic monitoring at the onset of sustained ventricular tachycardia. Wide QRS tachycardia with a heart rate of 160 beats per minute (bpm) was observed. |
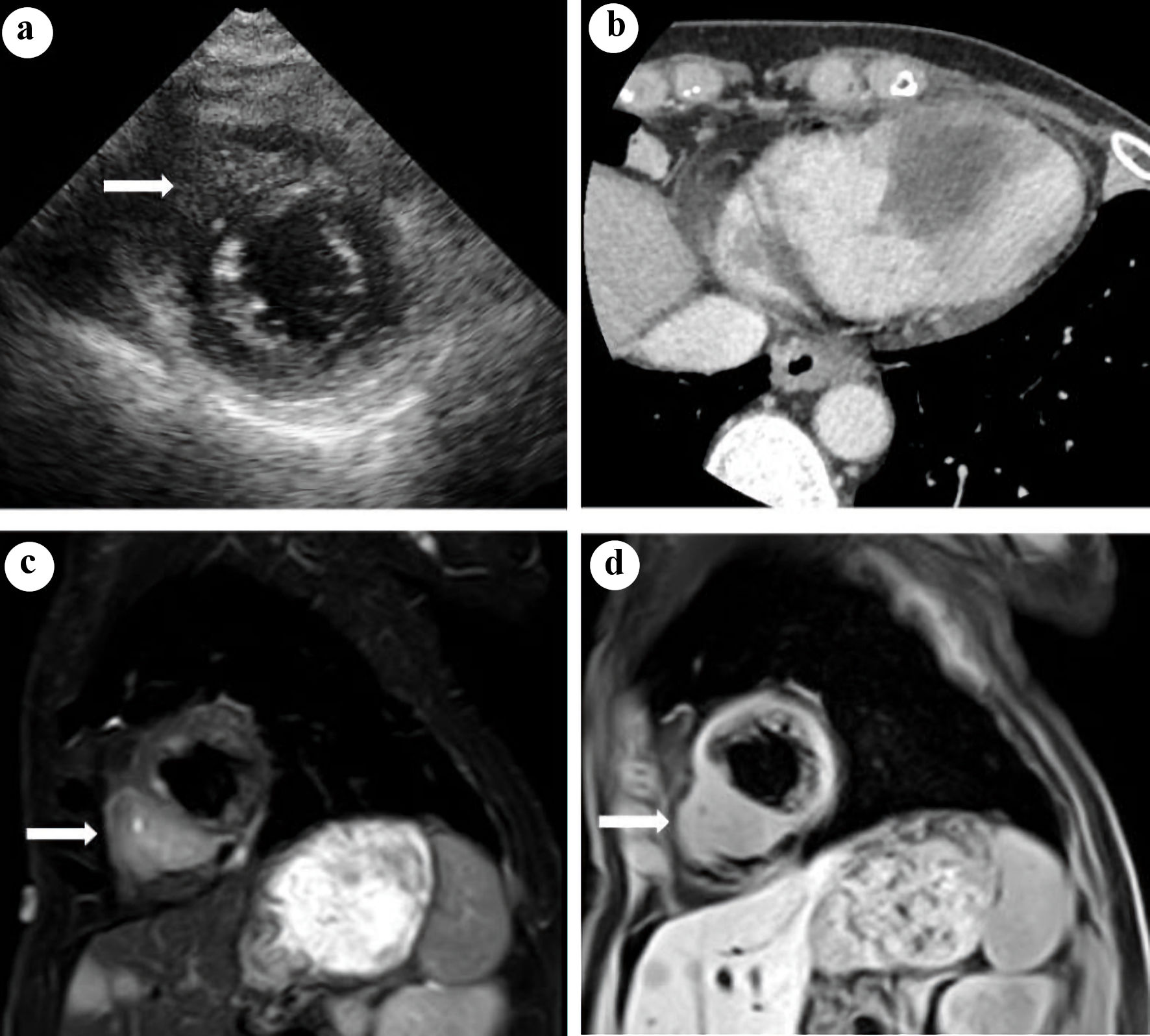 Click for large image | Figure 3. (a) Transthoracic echocardiography: a hypoechoic tumor in the right ventricular cavity was observed (arrow). (b) Contrast-enhanced computed tomography of the heart: a 35 × 33 mm hypodense tumor with indistinct borders was observed in the right ventricular wall and ventricular septum. Cardiac magnetic resonance imaging in a sagittal view: a tumor with high intensity on fat-suppressed T2-weighted images (c) and with isointensity to the myocardium on T1-weighted images (d) was observed in the right ventricular wall (arrow). |
Contrast-enhanced cardiac CT showed a 35 × 33 mm hypodense tumor in the right ventricular wall and ventricular septum (Fig. 3b). Cardiac magnetic resonance imaging (MRI) showed a tumor with high intensity on fat-suppressed T2-weighted images (Fig. 3c) and with isointensity to the myocardium on T1-weighted images (Fig. 3d) in the right ventricular wall. Fluorodeoxyglucose-positron emission tomography (FDG-PET)/CT performed in June 2018 showed a strong FDG accumulation with a maximum standard uptake value (SUVmax) of 6.14 in the right ventricular septum. Cardiac catheterization was performed, and coronary angiography showed no significant stenosis in the coronary arteries. Although myocardial biopsy was performed in the right ventricular wall, no malignant finding was detected. After a detailed examination of the abnormal findings on ECG performed upon admission, the patient was clinically diagnosed with right ventricular metastasis from lung cancer; however, pathological findings ware not obtained to confirm this diagnosis.
Treatment
Chemotherapy with carboplatin (AUC 5, day 1, every 3 weeks) plus oral S-1(100 mg/day, twice per day, day 1 - 14) was initiated. After discharge from the hospital, she received two courses of chemotherapy as outpatient treatment. However, intrapulmonary, hepatic, and adrenal metastases were observed; hence, she was judged to have progressive disease. In October of the same year, nivolumab treatment (240 mg/body, every 2 weeks) was started.
After three courses of nivolumab treatment, the patient was brought to the emergency department due to complaints of body malaise and increasing dyspnea intensity in November. The patient’s Glasgow Coma Scale score was 14 (E4V4M6); however, her pulse was difficult to palpate, and she was in a state of shock. An ECG performed at the time of visit showed sustained VT (heart rate: 160 bpm). Amiodarone was administered, but the patient did not show a positive response to the treatment. After delivery of cardioversion (biphasic, 100 J), sinus rhythm was restored. After the restoration of sinus rhythm, an ECG revealed the absence of QT prolongation, and TEE showed normal wall motion. The serum levels of cardiac troponin T (< 0.1 ng/mL) and creatine kinase (87 U/L) were normal. Serum electrolyte levels (Na: 132 mmol/L, K: 3.9 mmol/L, Cl: 98 mmol/L, corrected Ca: 9.6 mg/dL) and thyroid hormone levels (thyroid-stimulating hormone: 2.93 µIU/mL, free thyroxine: 1.33 ng/dL, free triiodothyronine: 2.51 pg/mL) were also in the normal range. She was treated with oral amiodarone and bisoprolol. However, the patient experienced frequent episodes of sustained VT with no triggers (Fig. 2b); delivery of cardioversion effectively terminated the sustained VT.
Follow-up and outcomes
The patient’s performance status (PS) worsened, making it difficult to continue pharmacotherapy for lung cancer. Hence, she only received palliative treatment. The patient’s condition gradually deteriorated, and she eventually died of cardiac arrest due to sinus bradycardia. Her family did not permit an autopsy.
| Discussion | ▴Top |
Cardiac metastasis of malignant tumors rarely occurs, and the primary cardiac metastatic neoplasms include lung cancer, breast cancer, malignant melanoma, malignant lymphoma, and leukemia. The frequency of cardiac metastasis is relatively high in patients with lung cancer than in those with other malignant tumors and has been reported to be 18.2-21.0% in autopsied patients with lung cancer [2]. The modes of cardiac metastasis include direct tumor invasion, hematogenous metastasis, retrograde lymphatic metastasis from mediastinal and hilar lymph nodes [5]. The most common type of cardiac metastasis is pericardial metastasis, while myocardial metastasis is infrequent, accounting for one-fifth of the cardiac metastases [6].
Since myocardial metastasis of malignant tumors lacks subjective symptoms, the diagnosis is sometimes delayed. In general, patients with myocardial metastasis of malignant tumors show a wide variety of symptoms such as dyspnea, cough, chest pain, and peripheral edema. Among them, arrhythmia and electrocardiographic abnormalities are considered to be the most frequent findings in patients with myocardial metastases [7]. This patient had no subjective symptoms when myocardial metastasis was diagnosed. However, ST depression observed on ECG was strong evidence to diagnose myocardial metastasis. With regard to electrocardiographic changes, ST-T changes are sometimes observed, as in this patient. However, low voltage and arrhythmias such as bundle branch block, atrioventricular block, extrasystole, and atrial fibrillation have also been reported, and findings differ depending on the site of metastasis [8]. Ischemic heart diseases, including myocardial infarction [9], Takotsubo cardiomyopathy [10], and myocardial injuries related to anticancer therapy must be differentiated in addition to myocardial metastasis if patients undergoing treatment for advanced lung cancer exhibit alterations in the ST-T wave complex. Anthracyclines and the anti-HER2 antibody, trastuzumab [11], are known to cause myocardial injuries related to anticancer therapy. Additionally, myocardial damages associated with radiation therapy [12] should be differentiated following the exposure of the cardiac tissue to radiation. Ischemic heart disease and cardiomyopathy were ruled out in the present case as echocardiography revealed good cardiac function and the absence of wall motion abnormality, and coronary angiography revealed the absence of coronary artery stenosis. Myocardial injuries related to anticancer therapy were ruled out as the patient did not receive any anticancer drug with a high risk of myocardial damage and the cardiac tissue was not exposed to radiation. The findings revealed that the changes in the ST-T wave complex were attributed to myocardial metastasis.
TTE is the quickest and easiest method to diagnose myocardial metastasis, and it can evaluate the location of tumors and extent of tumor invasion. However, it is sometimes difficult to differentiate myocardial metastasis from non-neoplastic diseases such as thrombosis. Therefore, more attention is required. A previous study also reported that localized wall thickening, decreased wall motion, and changes of echogenicity in the myocardium are findings of myocardial metastasis [13]. In terms of other diagnostic methods, contrast-enhanced CT is commonly used; MRI, FDG-PET/CT, technetium-99m myocardial scintigraphy are also useful in diagnosing metastasis. This patient’s tumor in the myocardium of the right ventricle was a hypodense tumor with indistinct boundaries on contrast-enhanced CT images and a tumor with high intensity on fat-suppressed T2-weighted MRI images. These findings were not inconsistent with the characteristics of intramyocardial metastasis. TTE is an easy and sensitive method that should be initially performed during the follow-up of patients with advanced lung cancer, followed by detailed examination with contrast-enhanced CT or MRI if any suspicion exists, for excluding cardiac metastasis.
Metastatic cardiac tumors are generally treated with chemotherapy and radiotherapy. Surgery is also performed in some cases. However, cardiac metastasis often occurs in patients with terminal stages. Therefore, surgical treatment is often difficult, even if cardiac metastasis is diagnosed before death. Cardiac metastasis of malignant tumors can cause heart failure, arrhythmia, and pericardial effusion. Several previous studies reported syncope and sudden death in patients with cardiac metastases [6, 14]; severe arrhythmia is considered to be one of the causes of death. Arrhythmia is very difficult to control, although antiarrhythmic drugs and catheter ablation are indicated in some patients [15]. In the literature we found four other cases of cardiac metastasis from lung cancer presenting with VT (Table 1) [16-19]. In these five cases, including our present case, four cases had previously or synchronously multiple distant metastases in addition to those in the heart. Cardiac metastases were detected in the ventricular wall or septum in the heart in all cases. Radiotherapy was performed in two cases. Although antiarrhythmic drugs were reported to be effective in these four cases, sustained VT was refractory to antiarrhythmic drugs, including amiodarone and bisoprolol, in the present case. In cases of recurrent VT, the prognosis may be extremely poor in patients who are refractory to antiarrhythmic drugs. In general, VT is classified as ischemic VT that develops along with ischemic heart disease such as myocardial infarction and non-ischemic VT due to other causes. Non-ischemic VT often accompanies cardiomyopathy or cardiac tumors. Additionally, the occurrence of myocardial injury related to anticancer therapy should be identified during cancer pharmacotherapy. Immune checkpoint inhibitors (ICIs) are being increasingly employed for the treatment of lung cancer, and immune-related adverse events (irAEs) can occur in any organ during treatment with ICIs. Although cardiac irAEs are rare, the occurrence of myocarditis, which is the main pathology, is often associated with arrhythmias such as complete atrioventricular block, atrial fibrillation, and VT, and has a poor prognosis [20]. In the present case, VT was observed following the administration of three courses of nivolumab; however, the levels of troponin T and creatine kinase were not elevated, and there was no evidence of myocarditis. Furthermore, an ECG showed monomorphic VT that had a left bundle branch block morphology, which indicated that the impulses originate in the right ventricle. The findings revealed that the development of VT was attributed to the exacerbation of right ventricular myocardial metastasis. However, the mechanism underlying the onset of arrhythmias in cardiac tumors remains to be clearly elucidated to date. A previous study reported the occurrence of VT accompanying cardiac tumors, which was thought to be caused by the formation of a reentrant circuit in the myocardium around or within the tumor [21]. Although an electrophysiological study or autopsy was not performed in the present study, it might be possible that the metastases in the right ventricular myocardium induced the electrophysiological abnormalities and development of VT, via a re-entry mechanism.
 Click to view | Table 1. Summary of the Reported Cases of Cardiac Metastasis Causing VT in Patients With Lung Cancer |
In conclusion, we here describe a rare case of primary squamous cell lung cancer with frequent episodes of sustained VT due to myocardial metastasis. In this patient, a drug-resistant sustained VT developed. Although cardioversion was effective, repeated treatments markedly deteriorated the patient’s PS. The prognosis is very poor when symptoms become evident; hence, the early diagnosis and appropriate treatment of cardiac metastasis by chemotherapy, cardiac radiotherapy, or surgery, are necessary prior to the development of symptoms in tolerant cases. Clinicians should pay attention to the development of cardiac metastasis in a patient with advanced lung cancer even without any symptoms.
Learning points
Arrhythmias and abnormalities in ECG are the most common diagnostic factors for myocardial metastases. The prognosis of myocardial metastases is generally poor once symptoms such as sustained VT appear. Furthermore, in cases of recurrent sustained VT, the prognosis may be extremely poor in patients who are refractory to antiarrhythmic drugs. In retrospect, myocardial metastasis could have been detected by follow-up examinations with contrast-enhanced CT or FDG-PET in addition to analyzing the alterations in the ECG. The early diagnosis and treatment of myocardial metastasis prior to the development of symptoms are crucial for improving patient prognosis.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained.
Author Contributions
ST, NF contributed mainly to the patient’s treatment. ST, NF, HI and KY drafted the manuscript. Images and description of CT, TTE and MRI results were prepared by ST and HI. All authors have read and approved the final manuscript.
Data Availability
The authors declare that the data supporting the findings of this case are available within the article.
Abbreviations
VT: ventricular tachycardia; ECG: electrocardiogram; TTE: transthoracic echocardiography; CT: computed tomography; MRI: magnetic resonance imaging; FDG-PET: fluorodeoxyglucose-positron emission tomography; SUVmax: maximum standard uptake value; EGFR: epidermal growth factor receptor; ALK: anaplastic lymphoma kinase; TPS: tumor proportion score; PD-L1: programmed cell death protein-1 ligand 1; AUC: area under the plasma concentration-time curve; PS: performance status; ICIs: immune checkpoint inhibitors; irAEs: immune-related adverse events
| References | ▴Top |
- Al-Mamgani A, Baartman L, Baaijens M, de Pree I, Incrocci L, Levendag PC. Cardiac metastases. Int J Clin Oncol. 2008;13(4):369-372.
doi pubmed - Bussani R, De-Giorgio F, Abbate A, Silvestri F. Cardiac metastases. J Clin Pathol. 2007;60(1):27-34.
doi pubmed pmc - Sheldon R, Isaac D. Metastatic melanoma to the heart presenting with ventricular tachycardia. Chest. 1991;99(5):1296-1298.
doi pubmed - Jaster M, Gutberlet M, Dinkloh N, Schneider P, Schultheiss HP, Morguet AJ. Ventricular tachycardia indicating cardiac involvement in metastatic leiomyosarcoma. J Clin Oncol. 2006;24(21):3502-3504.
doi pubmed - Tamura A, Matsubara O, Yoshimura N, Kasuga T, Akagawa S, Aoki N. Cardiac metastasis of lung cancer. A study of metastatic pathways and clinical manifestations. Cancer. 1992;70(2):437-442.
doi pubmed - Hanfling SM. Metastatic cancer to the heart. Review of the literature and report of 127 cases. Circulation. 1960;22:474-483.
doi pubmed - Waller BF, Gering LE, Branyas NA, Slack JD. Anatomy, histology, and pathology of the cardiac conduction system—Part IV. Clin Cardiol. 1993;16(6):507-511.
doi pubmed - Vallot F, Berghmans T, Delhaye F, Dagnelie J, Sculier JP. Electrocardiographic manifestations of heart metastasis from a primary lung cancer. Support Care Cancer. 2001;9(4):275-277.
doi pubmed - Daher IN, Luh JY, Duarte AG. Squamous cell lung cancer simulating an acute myocardial infarction. Chest. 2003;123(1):304-306.
doi pubmed - Desai A, Noor A, Joshi S, Kim AS. Takotsubo cardiomyopathy in cancer patients. Cardiooncology. 2019;5:7.
doi pubmed pmc - Volkova M, Russell R, 3rd. Anthracycline cardiotoxicity: prevalence, pathogenesis and treatment. Curr Cardiol Rev. 2011;7(4):214-220.
doi pubmed pmc - Lancellotti P, Nkomo VT, Badano LP, Bergler-Klein J, Bogaert J, Davin L, Cosyns B, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2013;14(8):721-740.
doi pubmed - Auger D, Pressacco J, Marcotte F, Tremblay A, Dore A, Ducharme A. Cardiac masses: an integrative approach using echocardiography and other imaging modalities. Heart. 2011;97(13):1101-1109.
doi pubmed - Seibert KA, Rettenmier CW, Waller BF, Battle WE, Levine AS, Roberts WC. Osteogenic sarcoma metastatic to the heart. Am J Med. 1982;73(1):136-141.
doi pubmed - Goldberg AD, Blankstein R, Padera RF. Tumors metastatic to the heart. Circulation. 2013;128(16):1790-1794.
doi pubmed - Leak D. Amiodarone for control of recurrent ventricular tachycardia secondary to cardiac metastasis. Tex Heart Inst J. 1998;25(3):198-200.
pubmed pmc - Li YY, Wang H, Cui YD, Liu HY, Yang JF. Sustained ventricular tachycardia secondary to cardiac metastasis of lung cancer. Chin Med J (Engl). 2018;131(3):352-353.
doi pubmed pmc - Kinoshita K, Hanibuchi M, Kishi M, Kanematsu T, Nishioka Y, Sone S. [Case of squamous cell lung cancer with myocardial metastasis complicated with ventricular tachycardia]. Nihon Kokyuki Gakkai Zasshi. 2009;47(9):817-822.
pubmed - Jumeau R, Vincenti MG, Pruvot E, Schwitter J, Vallet V, Zeverino M, Moeckli R, et al. Curative management of a cardiac metastasis from lung cancer revealed by an electrical storm. Clin Transl Radiat Oncol. 2020;21:62-65.
doi pubmed pmc - Palaskas N, Lopez-Mattei J, Durand JB, Iliescu C, Deswal A. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc. 2020;9(2):e013757.
doi pubmed pmc - Miyashita T, Miyazawa I, Kawaguchi T, Kasai T, Yamaura T, Ito T, Takei M, et al. A case of primary cardiac B cell lymphoma associated with ventricular tachycardia, successfully treated with systemic chemotherapy and radiotherapy: a long-term survival case. Jpn Circ J. 2000;64(2):135-138.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


